Credit: Public domain pictures.
Happy Easter everybody! It is that time of year again when you wake up excitedly on Easter Sunday and run into the garden to find the chocolate eggs the Easter Bunny hid for you! What? That’s just me? Hm. Well, in any case, you will probably have a couple of extra days off from work and this should be celebrated with a themed blog post! As you know, geodynamics is about the large scale dynamics of the Earth. One of the most important and most studied processes in geodynamics is mantle convection. However, convection processes are not only present on this large (mantle) scale. In fact, you can find convection processes everywhere around you. Think for example about adding cold milk to your hot tea or coffee: a more beautiful example of a(n analogue) model of convection you will only rarely find. However, for this happy Easter occasion, we will focus on the convection in eggs! Yes, your eyes do not deceive you: real eggs!
What is convection?
Let’s start with the definition of convection. Feel free to skip this section if you are a diehard geodynamicist already. For those of you that are not: convection is a natural process of heat transfer where material flows (convects) due to material density differences that are caused by a difference in temperature. To go back to our coffee analogy: the coffee is hot, and therefore less dense than the cold milk. If you pour the milk in the coffee, the milk is denser (~heavier) than the coffee and thus sinks to the bottom of your cup driven by buoyancy contrasts. This heat transfer via the movement of liquids is called convection. It happens in your coffee cup, and it also happens in the mantle of the Earth. And is an egg really that different? Imaging that the egg white is the mantle and the egg yolk the core and we have a nice analogy (okay, the yolk can move, I know. Cut me some slack).
Why would you study the convection in eggs?
Good question. I also didn’t have no clue at first. However, as it turns out, studying the convection in eggs is very important for the food processing of eggs. People like to cook with actual, intact eggs, but there is always a risk that raw eggs contain salmonellae. Apparently there are pasteurised ‘liquid egg’ products on the market, but they can not be used in as many different ways as real, intact eggs. So, in order to make sure that you can safely lick the spoon used to make the cake batter, the idea of ‘pasteurisation of intact eggs’ was born. By pasteurising intact eggs, the illness-inducing salmonellae is killed, without actually compromising the great versatility of the egg. In order to determine how long you should heat an egg at which temperature to kill all the salmonellae (and without accidentally boiling the egg), one needs to know how heat is transferred inside the egg. Hence, one should study the convection of an egg.
So what does convection in eggs look like?
Denys et al., 2004 conducted a numerical study into the computational fluid dynamics of conductive and convective heat transfer in eggs. They used three model setups:
• a reference egg without a yolk
• an egg with a yolk in the middle
• an egg with a yolk at the top
They looked at the time evolution of heating of the egg, while also keeping track of the coldest point in the egg (which is a measure of the pasteurisation process). The initial egg was at a uniform temperature of 24.5 ℃. They then simulated the placement of the egg in a 59.4 ℃ water bath. The calculated velocity fields and time evolution are shown in the figures below.
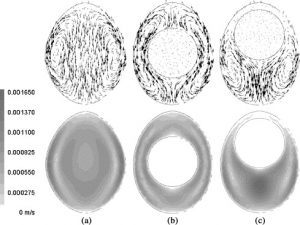
Calculated velocity field (top) and contours (bottom) after 30s of food processing in a water bath for the three types of model setups: a) no yolk, b) yolk in the middle, c) yolk at the top. Figure from Denys et al., 2004.
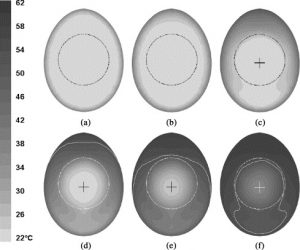
Time evolution of the temperature in the model of an egg with a yolk in the middle after being heated in a water bath for a) 5s, b) 10s, c) 30s, d) 80s, e) 150s, and f) 300s. White line is the 53 °C temperature contour and the cross represents the coldest point in the egg. Figure from Denys et al., 2004.
Denys et al., 2004 conclude that the convection in the egg changes the location of the coldest point in the egg: if there was only conduction at play, the coldest spot would be at the geometrical centre of the egg, but the convection forces the slowest heating zone to the bottom of the egg.
This conclusion is of course not applicable to the Earth: the core doesn’t move through the mantle, like the egg yolk can move through the egg white. Still, studying the process of convection systematically across all natural scales ultimately leads to a better understanding of the convection process, which is beneficial for everyone studying the physical process of convection on whatever scale.
So now you know what happens during convection in an egg. It’s not quite the Earth, but with a bit of festive imagination we can go a long way. Hopefully this piece of convection trivia will come in handy during the long Easter weekend. Enjoy!
Reference Denys, S., Pieters, J. G., & Dewettinck, K. (2004). Computational fluid dynamics analysis of combined conductive and convective heat transfer in model eggs. Journal of Food Engineering, 63(3), 281-290.