Assume you are under stress. What do you do? Take a walk in the park, order your favorite takeout, have a breakdown, or internally slip along preferred slip systems and develop a fabric? The response will mostly depend on what kind of material you are, how much stress you are under, and what environmental conditions you are subjected to. For instance, someone might listen to classical music after a stressful day at work and someone else might need heavy metal. One might just choose to breathe in and out if the stress is minor enough versus plan a vacation after a very stressful project deadline. One might take a walk if the weather is favorable or stay in and order food instead, if it is raining outside.
Yes, rocks flow!
Like us, minerals and rocks too respond to stress in different ways. Under low stress conditions, a mineral may have a very small change in its shape, i.e. strain after initial resistance. This change is reversible, i.e. the original shape is recovered once the applied stress is removed. As the applied stress is further increased, we go from the regime of elastic (reversible) deformation to that of a permanent deformation. If you are a rock rich in quartz and feldspars in the cold upper crust, you might fracture once the applied stress exceeds your strength, exhibiting a brittle behavior. You might also slip along those fracture planes and produce earthquakes. However, if you are an olivine crystal enjoying the higher temperatures of the upper mantle, then you might flow in a plastic manner.
Crystals have internal defects
A rock is made up of one or more kinds of minerals. Minerals are defined by their chemical compositions and the ordered internal arrangement of atoms, i.e. crystal structure. If we compute the force required to break the bond between atoms in a crystal, it is much larger than the force that can deform a crystal when measured in the lab. What is making these crystals weaker? Crystal defects – imperfections in the crystal lattice structure. Alas, nothing is perfect. Crystal defects might be point defects such as vacant spaces (vacancies) in the lattice structure, atoms occupying interstitial spaces, or impurities. Today, we will be focusing on the other kind of crystal defect though – line defects or dislocations (Figure 1).
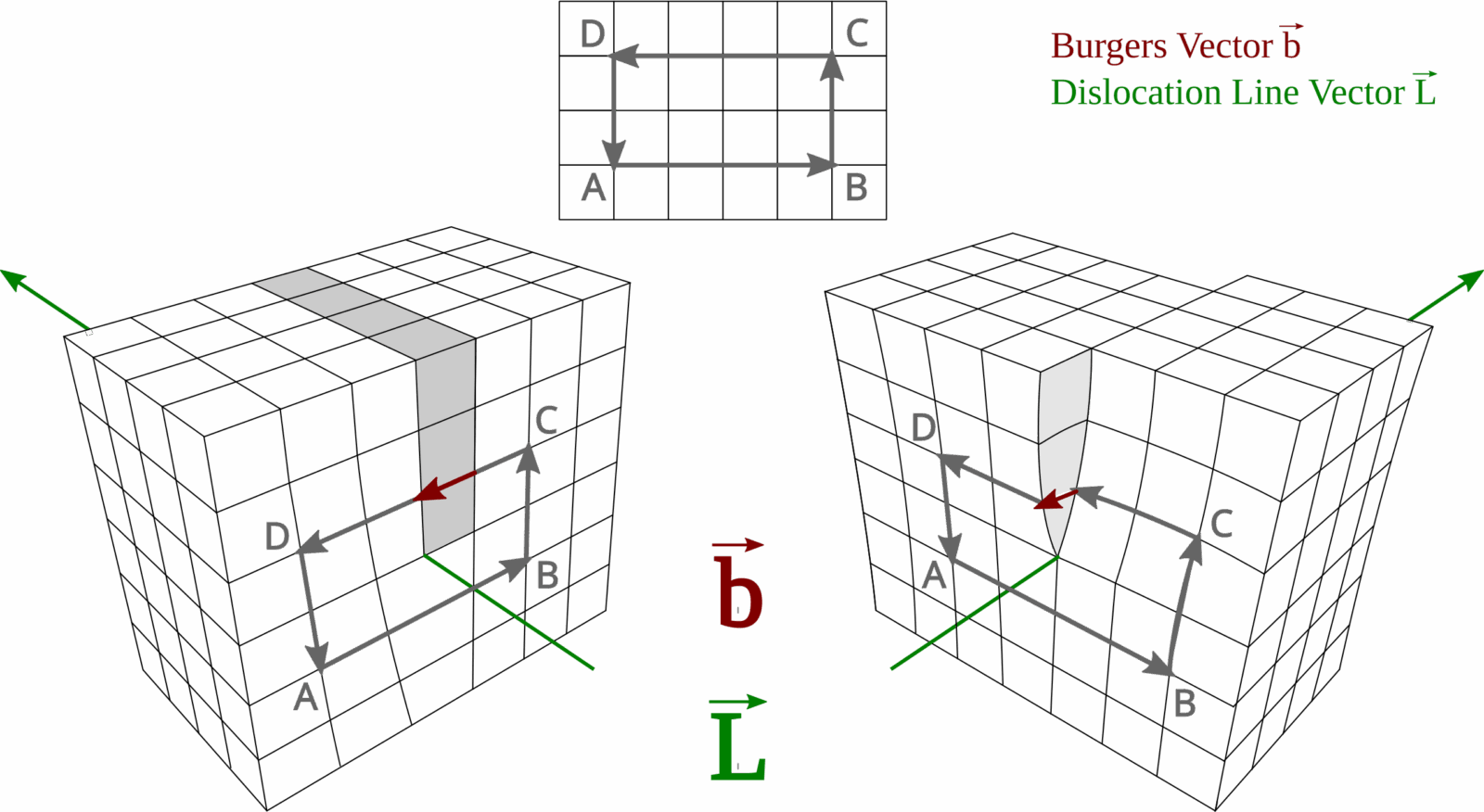
Figure 1. Edge (left) and screw (right) dislocation. (Image credit: Wikipedia, By Martin Fleck, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=96858277)
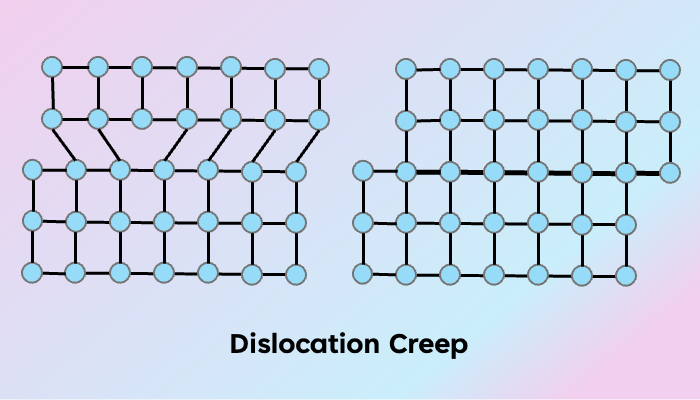
Dislocation creep
A dislocation represents the front along which slip occurs internally within a crystal grain. These intracrystalline slips usually occur on a specific crystallographic plane along a specific direction that can be dictated by the temperature-pressure conditions and the presence/absence of water (Karato, 2008). Much like whether one would prefer lying horizontally on their couch or walking vertically depending on what the day demands. The combination of a slip direction and a slip plane defines a slip system. The dislocation line may be parallel to the slip direction (screw dislocation) or perpendicular to it (edge dislocation) (Figure 1). Along the slip systems, the dislocation line can migrate through the crystal in a process called dislocation glide. Dislocations can also migrate out of the slip plane to another slip plane in what is called the dislocation climb. All of these dislocation motions come under dislocation creep. The migration of dislocations requires energy (like all chores) and therefore the slip system that can allow the movement of dislocations with the least energy is favored. Dislocation glide is more favored energetically than dislocation climb. The dislocation lines can continue moving until they go out of the crystal. Once the dislocations move out of the crystal, the line defect is removed, and the crystal shape is changed (Figure 2). This change in shape is very different from brittle deformation where the crystal would be fractured.
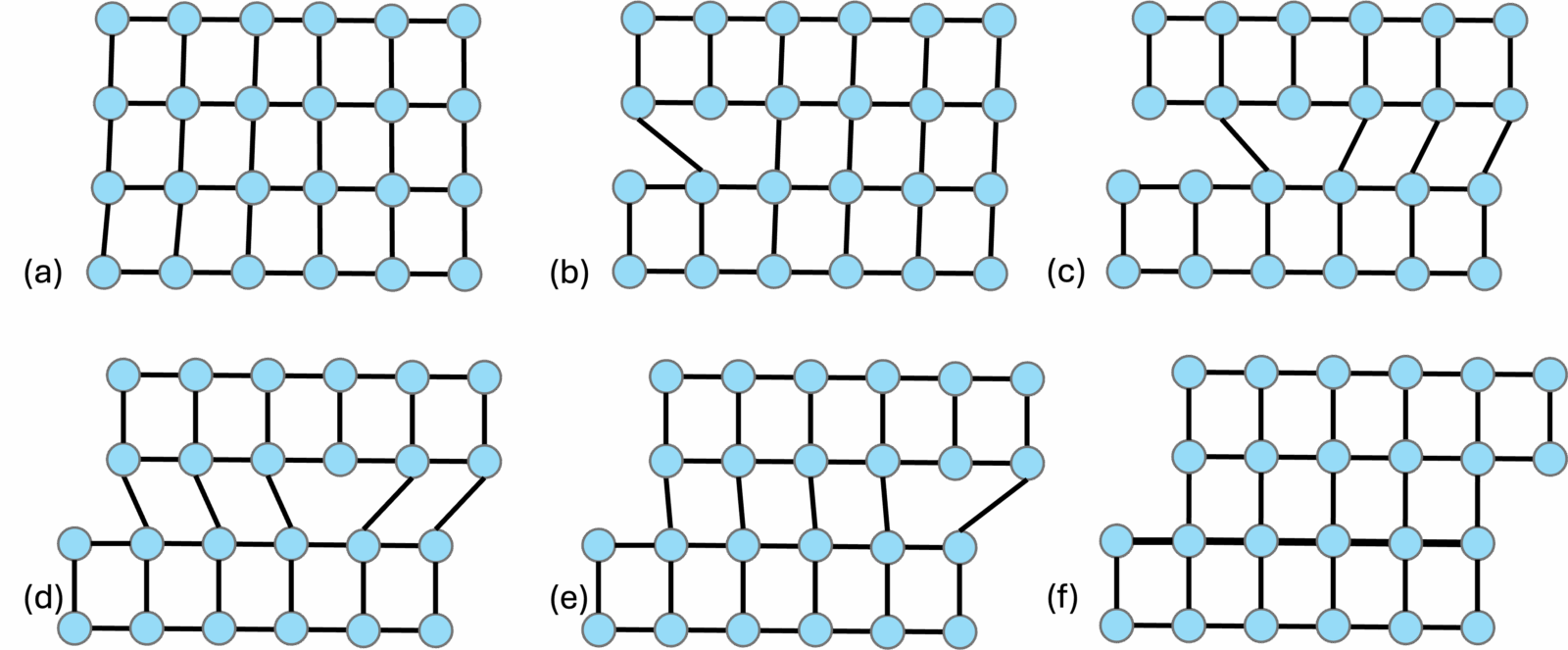
Figure 2. Deformation by dislocation creep: migration of an edge dislocation through a crystal lattice (adapted after Fossen, 2016).
Denser dislocations mean higher stresses
Dislocations disturb the atomic bonds around them and facilitate the movement of atoms. They also induce a stress field around them. Therefore, higher dislocation density in the crystal means that it is experiencing higher stress. Thermodynamically, the crystal prefers to be under lower stress (like most people), and this drives the dislocation motions in an attempt to get rid of the dislocations. The amount of shear stress required to move a dislocation within a crystal is quantified by Peierl’s stress. Peierl’s stress is an intrinsic material property which depends on the nature of bonds and the crystal structure. It is different for different slip systems.
Lattice-preferred orientation
Under the prevailing stress conditions, the slip system that allows dislocations to move at the expense of the least energy will be dominant. At suitable temperatures, the dominant slip system gets activated. The activation of a specific slip system also depends on the orientation of the crystals. The crystals deform by dislocation creep along the most favorable slip system. This results in a preferred orientation of the crystallographic axes and is called lattice-preferred orientation (LPO) or crystal-preferred orientation (CPO) (Figure 3). LPO is capable of providing valuable information about the stress state dominant during its formation. A very interesting example of LPO developed from dislocation creep is the previously mentioned olivine fabric in the upper mantle. The seismically fast crystallographic axes of olivine have been observed to align with plate motion or the orientation of orogenic belts in a number of shear wave splitting studies (Long & Silver, 2009). (See this related blog post)
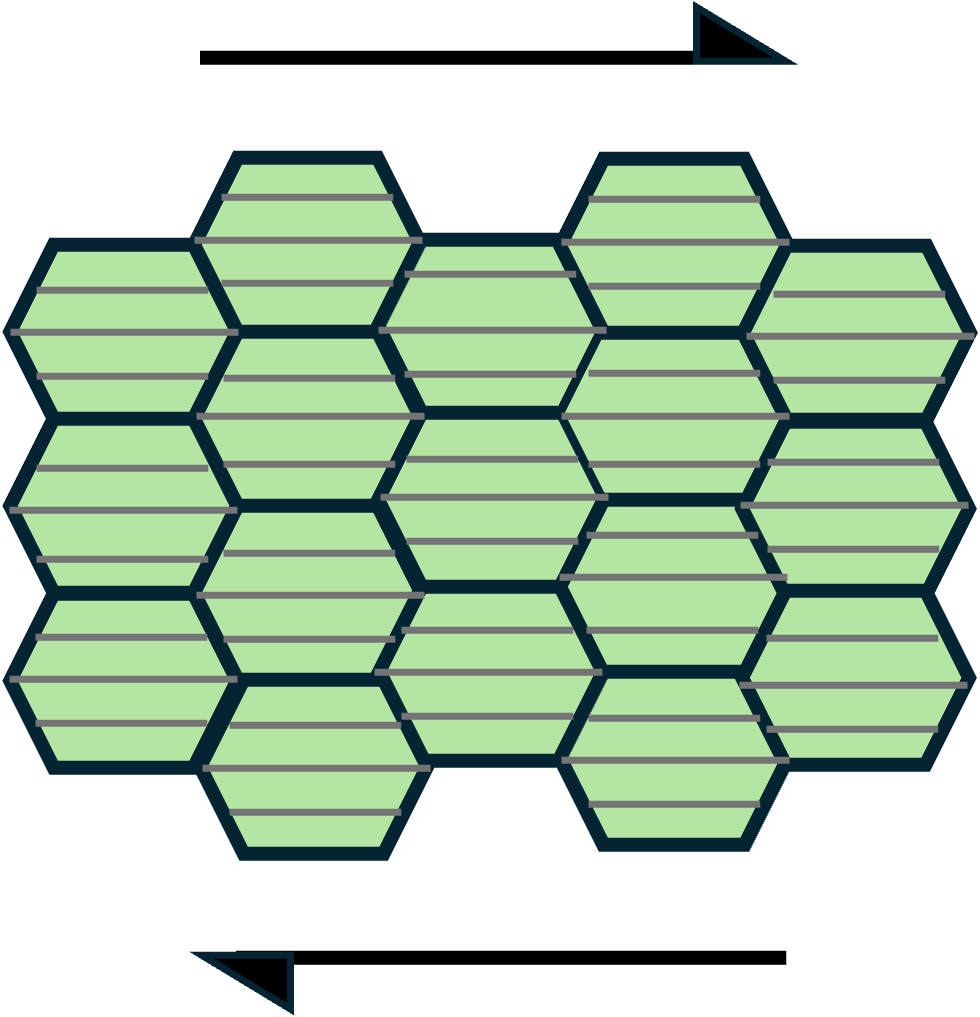
Figure 3. Lattice-preferred orientation due to dislocation creep along a favorable slip system (thin grey lines within each crystal).
What doesn’t kill you builds your fabric
Under high strain conditions, dislocation creep is an important process for creating mylonites. Mylonites are fine-grained, metamorphic rocks that have well-developed foliation and stretching lineation. Mylonites are formed in ductile shear zones and can preserve features representative of the sense of shear related to their formation. In these cases, one may as well say – what does not kill you builds your character fabric. Or alternatively, what does not kill you makes you stronger, at least in one direction.
References: Fossen, H. (2016). Structural Geology (2nd ed.). Cambridge: Cambridge University Press. Karato, S. (2008). Deformation of Earth Materials: An Introduction to the Rheology of Solid Earth. Cambridge University Press. https://doi.org/10.1017/CBO9780511804892 Long, M. D., & Silver, P. G. (2009). Shear Wave Splitting and Mantle Anisotropy: Measurements, Interpretations, and New Directions. Surveys in Geophysics, 30(4–5), 407–461. https://doi.org/10.1007/s10712-009-9075-1

