Earth’s oceans are not simply just water, they are a complicated multi-component fluid consisting of water and dissolved salts (ask anyone who has tried to drink it!). The existence of these salts has a significant impact on global ocean circulation. Nowhere is this more significant than in the polar oceans where it is one of the key factors influencing sea ice formation. In this week’s image of the week we are going to show you how freezing ocean water is a little more complicated than you may think!
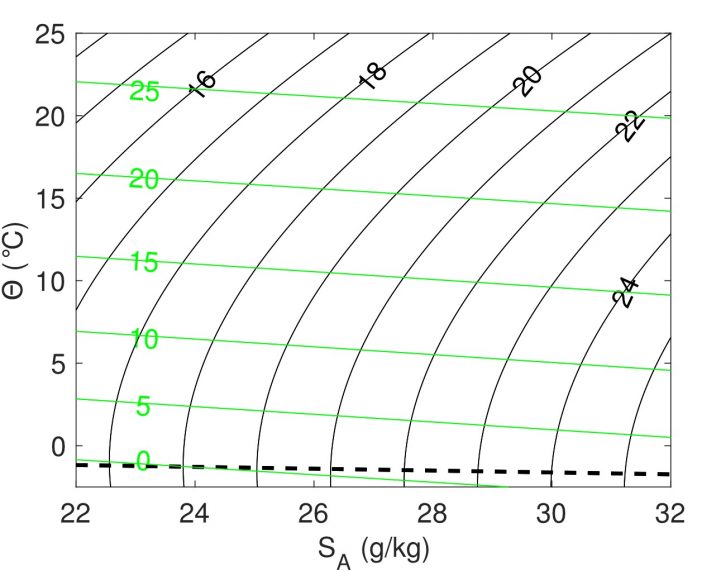
The salinity and temperature of ocean water affect its density; essentially how much it weighs. Typical ocean densities are around 1000 kg/m3 and, depending on the temperature and salinity may vary by up to 1 %. This seems tiny, but these small changes in density are what drive the thermohaline circulation, the dominant large-scale ocean circulation. The density of sea water, as a function of temperature and salinity, is expressed in terms of the equation of state (a mathematical way of describing the density of sea water in relation its temperature and salinity). Contours of the equation of state of seawater are shown in this week’s Image of the Week. The figure is from a recent paper by Mary-Louise Timmermans and Steven Jayne, who try to understand how changes in Arctic temperature will influence the density, and therefore the circulation, in the Arctic Ocean. The y-axis is temperature, and the x-axis is salinity. The black lines are density contours. The dashed line plots the freezing point of water.
Sea Ice Formation
Sea ice begins to form when ocean water is brought to this freezing point. If one was to put a cup of tap water into a freezer, ice would begin to form at 0 °C. But talk to a group of polar ocean modellers, and they will tell you the freezing point of water is about -1.8 °C. How can this be?
Let’s got back to our figure for some clues. Looking at the dashed line representing freezing point of ocean water you will notice that as the salinity increases, the freezing point decreases. So an increase in salinity of sea-water suppresses its freezing point. Just like how salt is used to melt ice in winter, it prevents the water from reaching its freezing point until the water reaches roughly -2 °C.
How does this all link together?
When the ocean gets cold, the influence of temperature on density changes, affecting how rapidly sea ice can form. Take a look at the bending of the black contours as the temperature is reduced to zero and below. Whereas in “normal”, warm contexts, a decrease in temperature leads to an increase in density, this changes as the temperature approaches 0 °C. As the ocean cools, the top-most, coldest water typically sinks, and is replaced by warmer water from below, driving ocean circulation convection. It therefore can take a long time to bring the surface of the ocean to near 0 °C. Since there is salt in the ocean, the water can reach colders temperatures where something very different happens. As the water continues to cool, the coldest water no longer sinks, and may even float, with sea-ice formation happening rapidly.
The formation process of sea ice, and its relationship to the ocean it forms out of is an extremely complicated and rich phenomenon, and it all depends on salt!
Further Reading
- Mary-Louise Timmermans and Steven R. Jayne, 2016: The Arctic Ocean Spices Up. J. Phys. Oceanogr. 46, 1277–1284, doi: 10.1175/JPO-D-16-0027.1.
- For more on sea ice check the National Snow and Ice Data Center (NSIDC) website – All About Sea Ice!
Edited by Emma Smith




