Sastrugi are significant features in glaciology, providing valuable insights into wind patterns, snow dynamics, and surface processes. So although at first sight they may be easy to walk over (quite literally), their patterns and features can tell us more than you might think, so next time take a moment to look and see the story they have to tell… The Song of Sastrugi The wind’s icy b ...[Read More]
You can’t unsee it – the impact of a good visual for scientific data
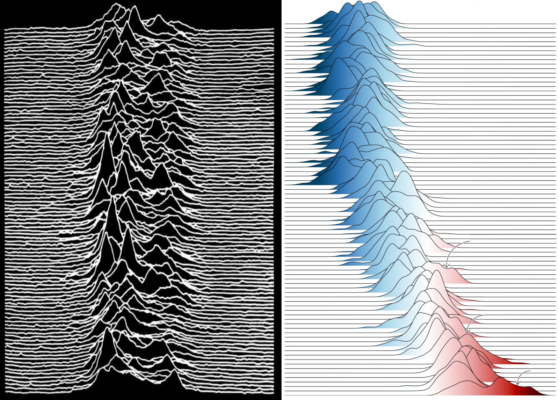
We are visual learners after all and for many of us, creating visual content is far more out of our comfort zone than the already hard earned skills of writing itself. Still, creating an accessible image can be pivotal to not only the success of your paper, but also the reach of your science in general. Today’s post started with a climate figure that went viral because of its similarity to the ico ...[Read More]
Did you know… about worms surviving in permafrost for at least 46000 years?
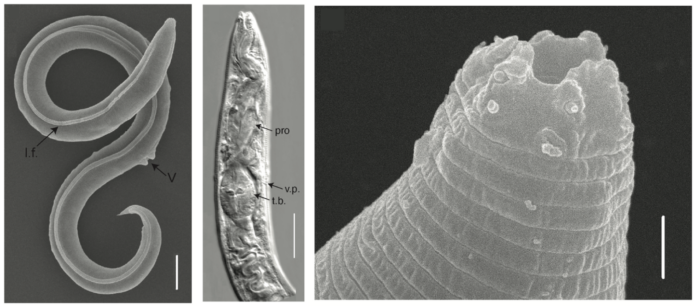
Lately permafrost makes the news more and more because of its enormous carbon stocks and its vulnerability to climate change. While permafrost greenhouse gas budget calculations are complex and harbour an ever-growing research community, its microbial ecology is still on the rise. A recent star are tiny roundworms that survived frozen in permafrost for 46’000 years. Take a short dip into this new ...[Read More]
We are back – with 4 Arctic fieldwork stories!
You might have missed our weekly blog posts, but we are back! This week’s post highlights four field work campaigns our cryo community conducted. Join us on a journey to Greenland, Svalbard and Alaska to learn about methane emissions, glacier flows, tundra fires and ice microbes. Chasing methane in Greenland The subglacial environment of the Greenland Ice Sheet is a relatively new discovered ...[Read More]
Image of the Week – Receiving messages from the deep

The Weddell Seal pops his head up through the hole in the floor of the shipping container… for the fourth time today. The shipping container is one of several making up our field camp on sea ice, 40 km from Scott Base – situated on Ross Island, in the south-western Ross Sea. Today I talk about the sub-ice platelet layer, which provides the base for a rich marine environment. Generating super ...[Read More]
Image of the week – The gaze of the ice cap
We are getting used to perceiving glaciers more and more distant and disconnected from our mountains. With each passing year, it is more difficult to observe them, reach them or climb them. They are becoming an exotic element of the Alpine imagination. When our gaze rests on a mountain glacier, with its crevasses and large moraines, we are filled with the fascination of someone observing a new nat ...[Read More]
Image of the week – Birds in the Arctic
I am a hobby ornithologist and love watching birds. It is fun and relaxing to search the trees or fields for feathered friends. One has to be aware of their surroundings, which can make birding even meditative. Here, I will tell you more about Arctic breeding birds, their population declines and I present to you three birds of the many I saw on my very first trips to the Arctic. Arctic breeding bi ...[Read More]
The intriguing order of cold terrains

Do you know what the periglacial environment is? Well, the word periglacial refers to those environments which are somehow sculpted by seasonal freeze and thaw cycles. The alternation of freezing and thawing conditions can change the landscape, creating some spectacular landforms. Stone circles are certainly among the most mysterious and fascinating. Come and discover them! Some definitions Glacia ...[Read More]
Taiwan’s Icy Past

The beautiful island of Taiwan (as given by its colonial name, Ilha Formosa) is primarily known for its lush tropical forests, delicious culinary cuisine, bubbling hot springs, and a bustling cityscape. But, what does Taiwan have to do with the cryosphere? Before you resist the urge to leave and get a bubble tea, read on to find out about Taiwan’s cryospheric past! From cities to mountains Taiwan ...[Read More]
More pancakes in the future!

More pancakes in the future – that sounds like a very good New Year’s Eve resolution for Sunday brunches, but it could also be a development of the most tasty looking sea ice shape in the Arctic. Let’s find out more! Arctic Sea Ice The growth and melt of Arctic sea ice follows a seasonal cycle. In the springtime, under the midnight sun, the sea ice begins to melt until it reaches its m ...[Read More]




