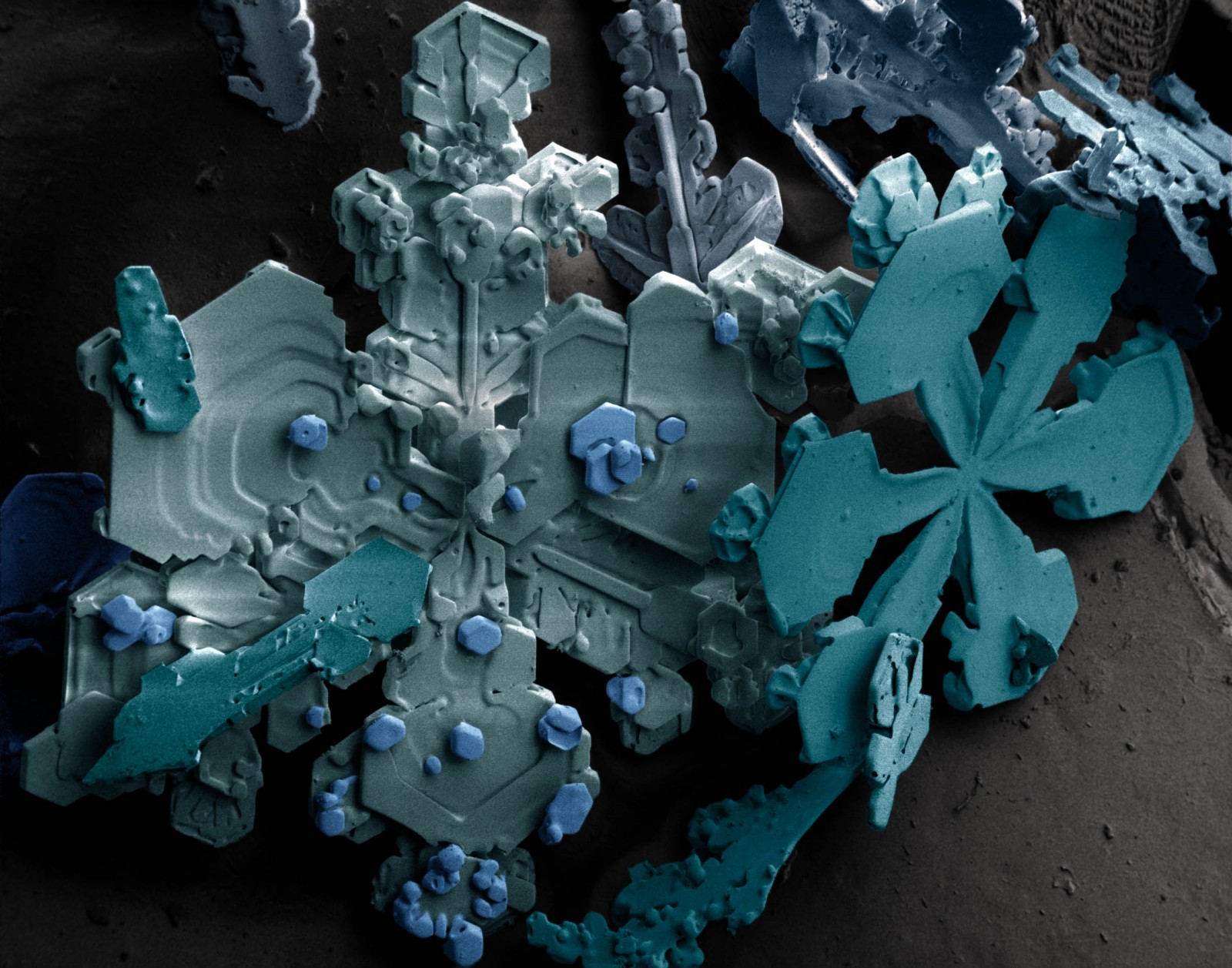
You remember last winter, when everything was white and you had so much fun building a snowman with your friends? What you see on this image above, is what you would see, if you took a tiny tiny piece of your snowman and put it under a low-temperature scanning electron microscope (SEM). The colours are called “pseudo colours”, they are computer generated based on the number of electrons reflected from a particular part of the image when scanned with a focussed beam of electrons. This is a standard technique used with SEM images to help identify patterns and structure in the image.
This week, let us take you on a journey…from the water vapour in a cloud to the snowman in your garden, to find out what leads to the complex structure you can see on our Image of the Week!
To this purpose we need to start at the very beginning, with a seemingly obvious question:
What is snow?
Snow is, very simply, precipitation in the form of ice crystals that originate in clouds. These ice crystals form when water vapour condensates directly to ice, without becoming liquid water.
From water vapour to ice crystals
For its formation, an ice crystal needs the atmosphere to be colder than the freezing point (0°C) and at least slightly humid. Additionally, like water droplets, ice crystals need a condensation nucleus, for example a dust or pollen particle, to start the growing process around – see the clip from Frozen Planet (below). During the transformation from water vapour to ice, an ice crystal always takes the form of a hexagon due to the way hydrogen and oxygen atoms bond to form water. Afterwards, the temperature and humidity of the atmosphere will shape the crystals and determine how fast they grow (see Fig. 2).
Between 0 and -60°C, the basic form (also called “habit”) of an ice crystal changes three times (near -3, -8 and -40°C). Between -3 and -8°C as well as below -40°C, the crystals take the form of six-sided plates, while between 0 an -3°C as well as between -8 and -40°C, the crystals form solid columns that are hexagonal in cross section.
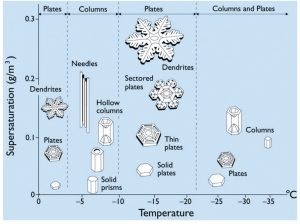
Fig2: Ice Crystal Morphology diagram, indicating the basic form of ice crystals as a function of temperature and supersaturation (humidity). For more information see Libbrecht (2005) [Credit: K. Libbrecht]
From ice crystals to snowflakes
When an ice crystal becomes heavier than the surrounding air, it falls down. If it meets other ice crystals on its way to the ground, they will aggregate, forming new structures and the snowflake will grow. When reaching the ground, a snowflake can therefore be an aggregate of hundreds or sometimes even thousands of ice crystals.
From snowflakes to snowmen
Even when the snowflake has reached the ground, the journey is not finished. If the snow does not melt away rapidly, ice crystals will still modify their texture, size, and shape due to changing temperature and moisture conditions, melting and refreezing processes and/or compressing due to subsequent snowfall.
When the snow cover grows due to many subsequent snowfalls (over the winter or in cold areas over several years), a complex layered structure forms, made up of a variety of ice crystals, that reflect both the weather conditions at the time of deposition and the changes within the snow cover over time.
OR… you build a snowman out of it 🙂
![Olaf the enchanted snowman from Disney's 2013 animated feature film, Frozen. [Credit : Disney Wikia]](https://blogs.egu.eu/divisions/cr/files/2016/09/Olaf_transparent_pose-2.jpg)
Olaf the enchanted snowman from Disney’s 2013 animated feature film, Frozen. [Credit : Disney Wikia]
- How snow forms – National Snow and Ice Data Center (NSIDC)
- How do snowflakes form? – Hobart King for geology.com
- “No Two Snowflakes the Same” Likely True, Roach, J. (2007), National Geographic News
- Snowcrystals.com – Kenneth G. Libbrecht, professor of physics at the California Institute of Technology
- Libbrecht, K. G. (2005) The physics of snow crystals. Reports on Progress in Physics, 68 (4). pp. 855-895.
- Wallace, J.M. and Hobbs, P.V. (2006), “Microphysics of Cold Clouds” in Atmospheric Science : An Introductory Survey., 2nd edition, Academic Press, p. 504
Edited by Emma Smith and Sophie Berger


Pingback: GeoLog | Announcing the winner of the EGU Best Blog Post of 2016 Competition
Pingback: Tiedehaiku: jää // Sciku: ice – Vihreäkiven arvoitus