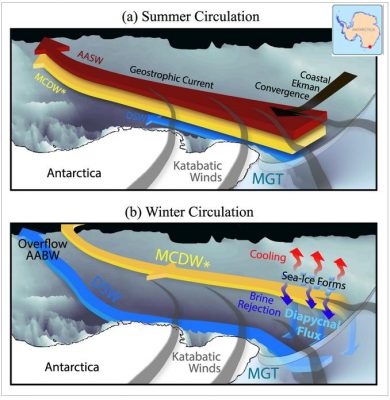
Earth’s oceans are not simply just water, they are a complicated multi-component fluid consisting of water and dissolved salts (ask anyone who has tried to drink it!). The existence of these salts has a significant impact on global ocean circulation. Nowhere is this more significant than in the polar oceans where it is one of the key factors influencing sea ice formation. In this week’s imag ...[Read More]
Image of the week – Our salty seas and how this affects sea ice growth
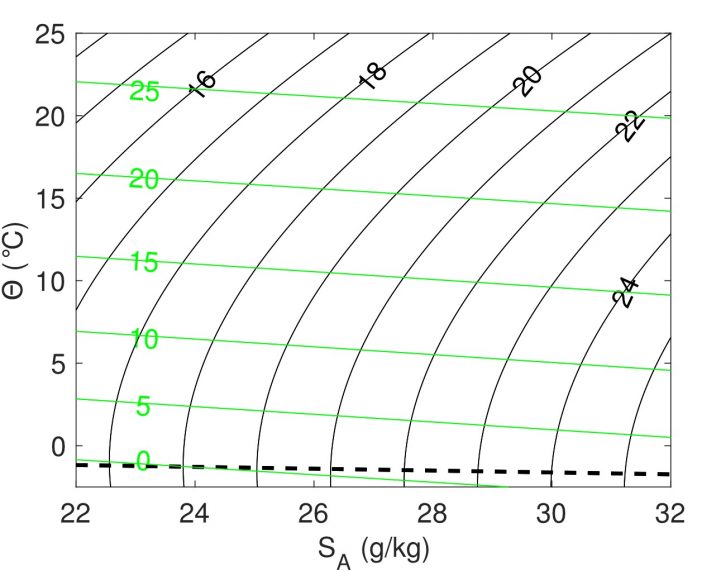
Lines of constant density for sea water . Black lines show lines of constant density as a function of temperature (y-axis) and salinity (x-axis). The dashed black line indicates, for a given salinity, the temperature at which the water will freeze. Figure adapted from Timmermans and Jayne (2016) - with permission.