“The more any indivisible exceeds, the heavier it is”.
Democritus (c. 460 – c. 370 BC).

Modern desiccation cracks in blue clay derived from the early Cambrian Lontova Formation near Kunda in Estonia. Credit: Wikipedia user Pikne. This file is licensed under the Creative Commons Attribution-Share Alike 2.5 Generic license.
What is clay?
Clays are particles. Very, very small mineral particles. You cannot see them, you cannot handle them… but unity makes strength. Clays are one of the major mineral components of the soil, whose chemical and physical properties depend on them. The study of soil clay minerals allows a general idea of composition and genesis of soils.
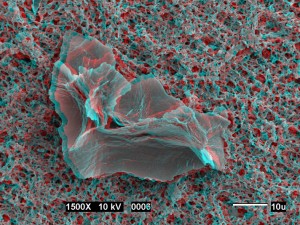
Stereo image of large soil smectite quasicrystal on cellulose filter paper. Do not tell me you threw your 3D glasses away …. Photo courtesy David Laird, USDA, ARS, National Soil Tilth Laboratory. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.
Since ancient times it is known that some soil components are able to exchange nutrients. If a small amount of clay is submitted to electrolysis, silica, aluminium and iron oxides accumulate in the anode. On the other hand, K+, Na+, Ca2+ and Mg2+ and other cations will accumulate in the catode.
Structure of clays
From the chemical point of view, clays are the most important component of the soil mineral fraction, as it is constituted by charged particles able to interact with the soil solution. Clay minerals are aluminosilicates. These are basically constituted by Si, Al and O, as well as other elements such as Na, K, Ca, Mg or Fe in variable proportions. Clays are a group of secondary minerals (minerals formed by the subsolidus alteration of a pre-existing primary mineral, may be a mineral in an igneous rock or another clay mineral) after chemical weathering. Clays have a colloidal size (below 2 microns), with well-defined crystal structure and a large surface/volume ratio.
Small balls?
However, one of the things that students have a harder effort to understand when they first meet with clay, is that they are not balls. They have multiple forms!
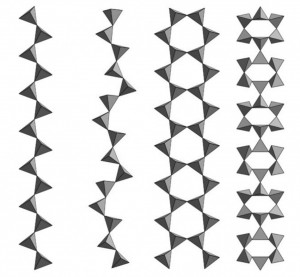
All silicates are constituted by common structural units, Si-O tetrahedrons. Silicon is located in the center of the tetrahedron and surrounded by 4 oxygens at the corners. This group is electrically unbalanced ((SiO4)4-), so that the oxygen atoms are combined with other cations to compensate their negative charges. The number of vertices shared by each tetrahedron may be 0, 1, 2, 3 or 4). Tetrahedra may also join by their bases to form hexahedral molecules ((Si2O4)n2n-). Depending on the number of oxygens that coordinate with other silicon cations, large groups of silicates may form in different shapes:
- No oxygens shared: isolated molecules (nesosilicates or orthosilicates)
- One oxygen shared: pairs (sorosilicates)
- Two oxygens shared: rings (cyclosilicates)
- Two-three oxygens shared: chains (inosilicates)
- Three oxygens shared: planes (phyllosilicates)
- Four oxygens shared: tridimensional structures (tectosilicates)
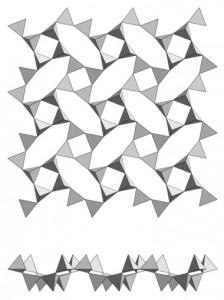
You can see some examples in the following figures:
Complicated? Different minerals may be formed in each major group of silicates depending on the number of oxygens binding cations other than silicon. Basically, the structure of these minerals is a repetition of a unit cell formed by the association of tetrahedra (isolated or in groups) and the cations are located between the tetrahedral groups.
Phyllosilicates
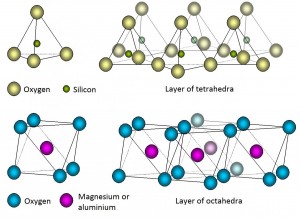
Phyllosilicates are the most important group in soils. Phyllosilicates form by groups of tetrahedral sharing three vertices. The fourth vertex binds to the central cation of an octahedron, usually magnesium or aluminum. Thus, the structure of these minerals is composed of a stack of layers of tetrahedra and octahedra, forming lamellar structures, with layers linked by shared oxygens. Consequently, layers are intimately bonded and are very difficult to separate.
According to the repeating pattern, two types of films with different structures are formed:
- the 1: 1 structure, with a tetrahedral layer and another octahedral, and
- the 2: 1 structure, with two layers of tetrahedrons enclosing one layer of octahedra.
Central positions in the tetrahedral and octahedral units (usually Si, Mg or Al) may be substituted with other cations, resulting in changes of the global charge. Depending on these substitutions, the layer where they occur and the cations associated to resulting charges, different mineral species will appear. Here you are some of them:

Smectite, trioctahedral. Credit: Anthony Priestas, Boston University. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.

Smectite, trioctahedral. Credit: Anthony Priestas, Boston University. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.
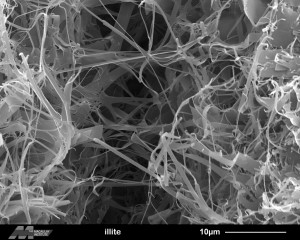
Illite, fibrous. Credit: Evelyne Delbos, James Hutton Institute. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.

Dickite. Credit: Evelyne Delbos, James Hutton Institute. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.
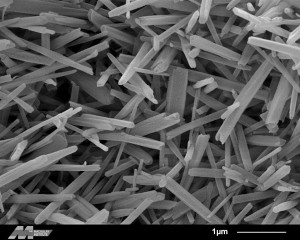
Halloysite. Credit: Evelyne Delbos, James Hutton Institute. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.
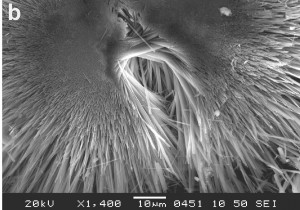
Mordenite. Credit: Hülya Kaçmaz, Dokuz Eylul University, Buca-Izmir/Turkey. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.

Chlorite, Fe-Al-rich. Credit: Michal Skiba, Institute of Geological Sciences, Jagiellonian University. Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.

Halloysite (sphaeroidal). Credit: Ömer Işik Ece, Istanbul Technical University . Click to see the original source at the ‘Images of Clay Archive’ of the Mineralogical Society of Great Britain & Ireland and The Clay Minerals Society.
Read more
- Brigatti MF, Galan E, Theng BKG. 2006. Structures and mineralogy of clay minerals. In: Bergaya F, Theng BKG, Lagaly G (Eds.), Developments in clay science. Vol. 1. Handbook of clay science. Elsevier. Amsterdam.
- Landais P. 2013. Overview of the clay mineralogy studies presented at the ‘Clays in natural and engineered barriers for radioactive waste confinement’ meeting, Montpellier, October 2012. Clay Minerals 48, 149.
- Meunier A. 2005. Clays. Springer. Berlin.
- Mukherjee S. 2013. The science of clays. Springer. Berlin.
This post has been also published in gsoil.wordpress.com.