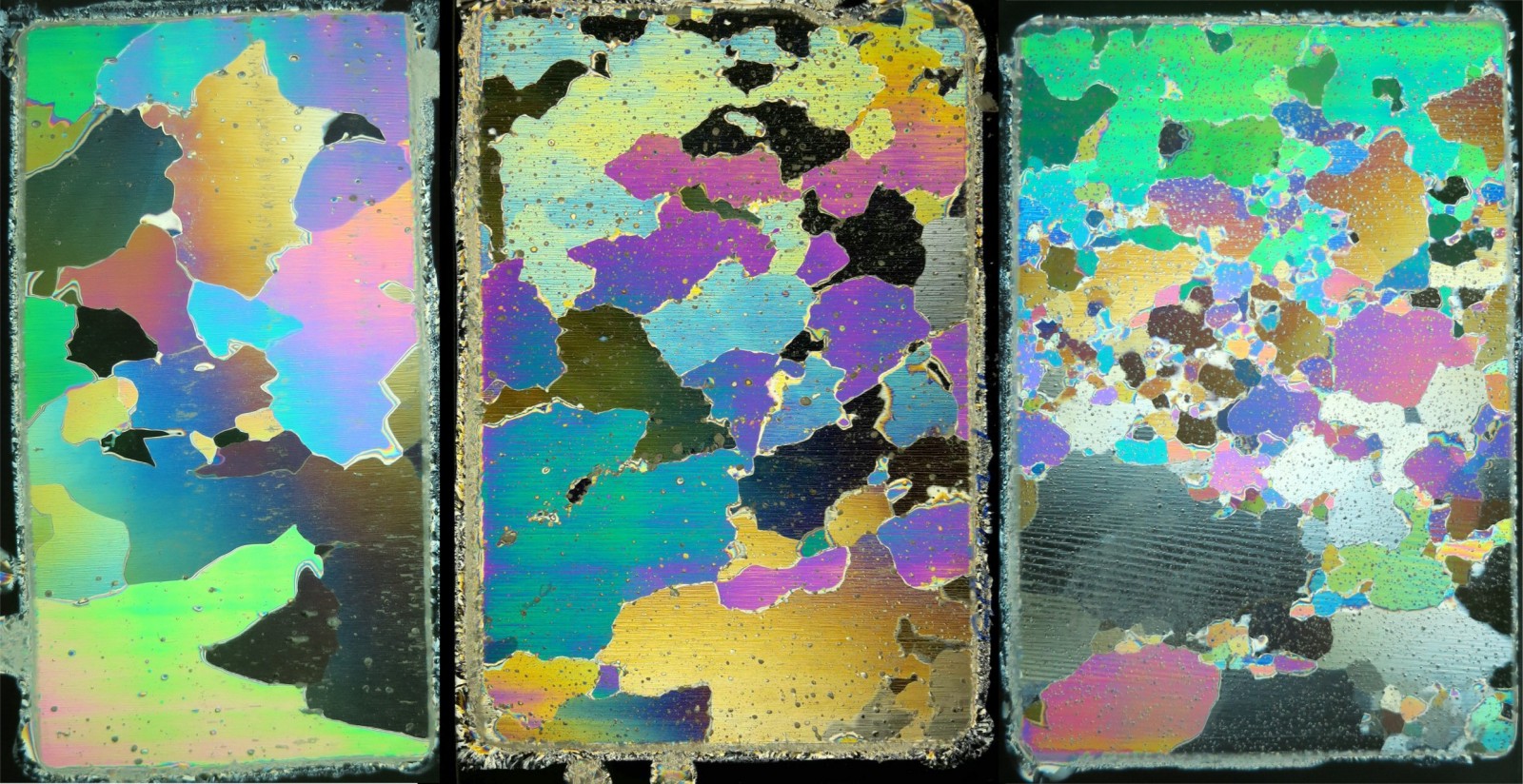
When you think of glacier ice, what colour first springs to mind? Maybe white, blue or transparent? Well, glacier ice can, in fact, be mesmerising and multi-coloured! Our image of the week shows thin sections of glacier ice under polarised light. These sections were cut from block samples of two Alpine glaciers in Switzerland (Chli Titlis and Grenzgletscher).
In these images the individual ice crystals (Fig. 1 ) can be easily distinguished due to the different colours (see previous post about sea ice) and most of them are large (Fig. 1 ) due to the relatively high temperature of the glaciers they originate from; ice crystals grow faster at high temperatures, close to zero!
Now we know the answer to “what is the colour of ice?” can not be simply answered with “transparent”, the obvious follow-up question is:
Why is ice colourful?
While ice is, of course, transparent (Fig. 2 ) – when we see it as icicles on the roof, as fern frost on a window or as ice cubes in our gin and tonic, it can have any colour – if you look at it in special light – polarised light (Fig. 3 ).

Figure 2: A thin section of ice (~0.3 mm thick) appears transparent under normal light conditions [Credit: Johanna Kerch]
Linearly polarised light is produced by putting a filter in front of a light source. Before being polarised, the light is an electromagnetic wave that vibrates in many directions. The polarising filter, which looks a bit like a very small picket fence, only lets light through that vibrates in the direction of the “gaps in the fence”. If we have two such filters and put them in a row, but rotate the second filter by 90° no light will come through because polarised light from the first filter will not fit through the gaps at the second filter. However, if we put a very thin slice of glacier ice between the two filters we begin to see the colours!
This effect can be observed because ice is birefringent. This means, that light travelling through the ice is split into two parts by the crystal structure of the ice. To help you understand, we have created this analogy: imagine a pair of children who enter a forest side-by-side and hand-in-hand, but they split up to travel through the forest. One part of the light (one child) can travel faster than the other because, it is interacting less with the crystal lattice (less dense part of the forest) . At the end of their separated journey through the ice sample the two parts of light recombine (children are hand-in-hand again), but because they were travelling at different speeds they will be out of phase, meaning the recombined light will have a different polarisation than it did when it entered the ice after passing through the first polariser (one child will be a bit behind the other, rather than side-by-side). Only in case where the new polarisation is 90° rotated can the light pass through the second filter.
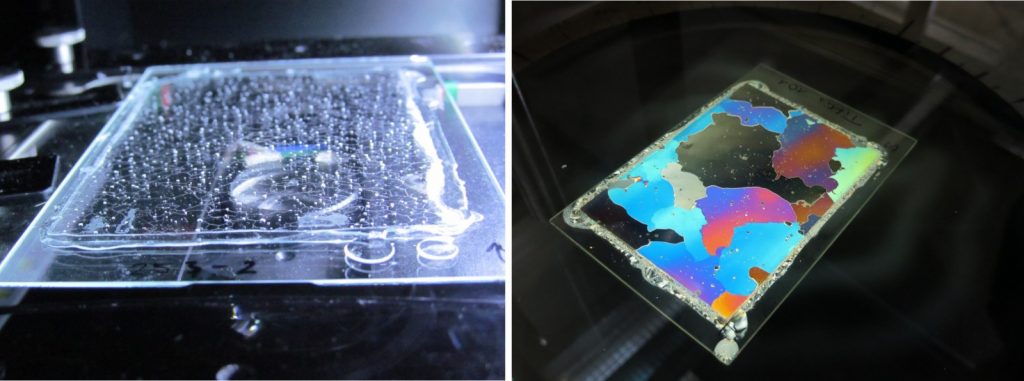
Figure 3: Left: transparent ice thin section (0.3 mm thick) on a glass plate during measurement viewed from the side without polarisers. Right: thin section between two polarisers shows crystals in ice section in different colours [Credit: Johanna Kerch].
However, it gets a bit more complicated, white light is a collection of lots of different waves with different wave lengths, which corresponds to different colours (shorter wave lengths are bluish, longer wave lengths are reddish and in between there is yellow-green). Each of these wave lengths is split up (as described above) when entering the ice sample. So each wave length has two waves travelling with different speeds (imagine a whole group of children who arrive at the forest in pairs, hand-in-hand, forced to split up to go through the forest single file). After exiting the ice sample, the two parts for each wave length recombine (children are back in pairs), and each pair of of waves, at a given wave length has a new polarisation direction. Not all of them can pass the second filter, only those wave lengths where the new polarisation is 90° rotated. Therefore, instead of white light only light of specific colours completes it’s journey through the second filter, to be seen by the observer – all the other colours are swallowed (all the children that don’t make it are eaten by wild animals in the forest!!). Because different crystals in a slice of glacier ice are oriented in various directions, they exert different amounts of birefringence on the light passing through them, this means they appear in different colours when viewed through the second polarising filter (Fig. 3 ). So…that’s cool and allowed us to make a wild analogy about children in a forest, but why is this scientifically useful?
Polarisation Microscopy
The technique by which we examine the ice between crossed polarisers to map the different crystals is called polarisation microscopy. The multi-coloured images of thin ice slices allow us to understand the orientation of the individual crystals, which is important to understand the mechanical properties of glacier ice – but this is another story, for another blog post.
Right, now we have to go and rescue some children from a forest!
Further Reading
- On polarised light phenomena: Polarized Light by Dennis H. Goldstein
- Previous blog post on the colour of icebergs
Personal note on outreach:
From my experience in the ice laboratory most people, especially children, are immediately captured by the birefringence effect in ice. It’s a great starting point to get them interested in glaciological issues!
Edited by Emma Smith
 Johanna Kerch is a postdoctoral researcher at Alfred-Wegener-Institute in Bremerhaven. Her research focus is on crystal-preferred orientation and microstructure of glacier ice and how it links to other physical properties in ice and the deformation mechanisms in glacier ice. She has studied cold and temperate glacier ice from various sites in the Alps and has recently been involved in making measurements of the physical properties of the EGRIP ice core. She tweets as @JohannaKerch.
Johanna Kerch is a postdoctoral researcher at Alfred-Wegener-Institute in Bremerhaven. Her research focus is on crystal-preferred orientation and microstructure of glacier ice and how it links to other physical properties in ice and the deformation mechanisms in glacier ice. She has studied cold and temperate glacier ice from various sites in the Alps and has recently been involved in making measurements of the physical properties of the EGRIP ice core. She tweets as @JohannaKerch.




