
New research shows that natural accumulations of carbon dioxide (CO2) that have been trapped underground for around 100,000 years have not significantly corroded the rocks above, suggesting that storing CO2 in reservoirs deep underground is much safer and more predictable over long periods of time than previously thought, explains Suzanne Hangx a postdoctoral researcher at the University of Utrecht.
The findings, published today in the journal Nature Communications, demonstrate the viability of a process called carbon capture and storage (CCS) as a solution to reducing carbon emissions from coal and gas-fired power stations, say researchers.
About 80% of the global carbon emissions emitted by the energy sector come from the burning of fossil fuels, which releases large volumes of CO2 into the atmosphere, contributing to climate change. With the growing global energy demand, fossil fuels are likely to continue to remain part of the energy mix. To mitigate CO2 emissions, one possible solution is to capture the carbon dioxide produced at power stations, compress it, and pump it into reservoirs in the rock more than a kilometer underground. This process is called carbon capture and storage (CCS). The CO2 must remain buried for at least 10,000 years to help alleviate the impacts of climate change.
The key component in the safety of geological storage of CO2 is an impermeable rock barrier (the ‘lid’ or caprock) over the porous rock layer (the ‘container’ or reservoir) in which the CO2 is stored in the pores – see Figure X. Although the CO2 will be injected as a dense fluid, it is still less dense than the brines originally filling the pores in the reservoir sandstones, and will rise until trapped by the relatively impermeable caprocks. One of the main concerns is that the CO2 will then slowly dissolve in the reservoir pore water, forming a slightly acidic, carbonated solution, which can only enter the caprock by diffusion through the pore water, a very slow process.
Some earlier studies, using computer simulations and laboratory experiments, have suggested that caprocks might be progressively corroded as these acidic, carbonated solutions diffuse upwards, creating weaker and more permeable layers of rock several meters thick and, in turn, jeopardizing the secure retention of the CO2. Therefore, for the safe implementation of carbon capture and storage, it is important to accurately determine how long the CO2 pumped underground will remain securely buried. This has important implications for regulating, maintaining, and insuring future CO2 storage sites.
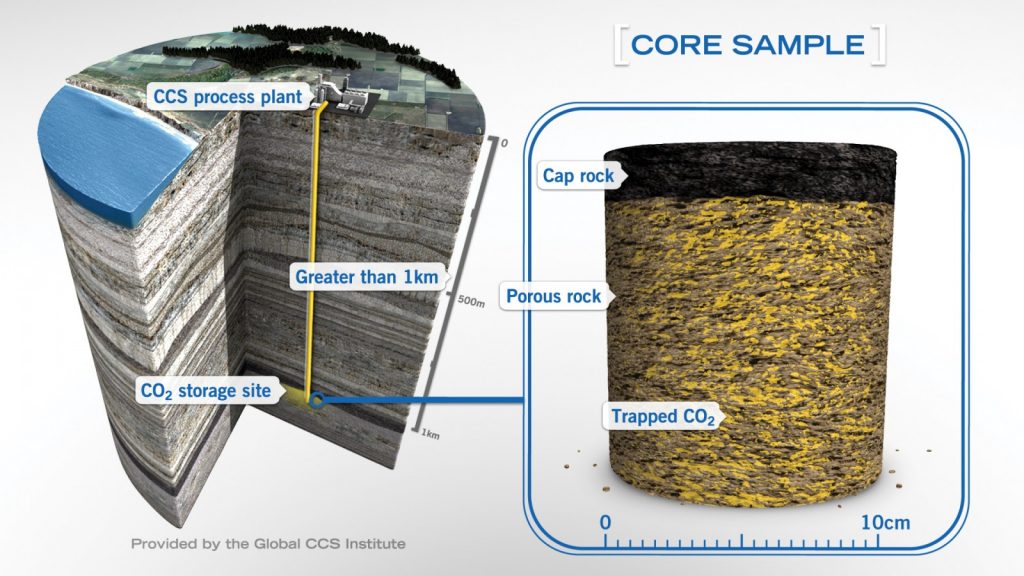
Schematic diagram of a storage site showing the injection of CO2 (in yellow) at a depth of more than one kilometer into a layer of porous rock (the ‘container’ or reservoir), and kept from moving upwards by a sealing layer (the ‘lid’ or caprock). Via Global CCS Institute
To understand what will happen in complex, natural systems, on much longer time-scales than can be achieved in a laboratory, a team of international researchers and industry experts traveled to the Colorado Plateau in the USA, where large natural pockets of CO2 have been safely buried underground in sedimentary rocks for over 100,000 years. The team drilled deep below the surface into one of the natural CO2 reservoirs in a drilling project sponsored by Shell, to recover samples of these rock layers and the fluids confined in the rock pores.
The team studied the corrosion of the rock by the acidic carbonated water, and how this has affected the ability of the caprock to act as an effective trap over long periods of time (thousands to millions of years). Their analysis studied the mineralogy and geochemistry of the caprock and included bombarding samples of the rock with neutrons at a facility in Germany to better understand any changes that may have occurred in the pore structure and permeability of the caprock.
They found that the CO2 had very little impact on corrosion of the caprock, with corrosion limited to a layer only 7cm thick. This is considerably less than the amount of corrosion predicted in some earlier studies, which suggested that this layer might be many metres thick. The researchers also used computer simulations, calibrated with data collected from the rock samples, to show that this layer took at least 100,000 years to form, an age consistent with how long the site is known to have contained CO2. The research demonstrates that the natural resistance of the caprock minerals to the acidic carbonated waters makes burying CO2 underground a far more predictable and secure process than previously estimated. With careful evaluation, burying carbon dioxide underground will prove safer than emitting CO2 directly to the atmosphere.
By Suzanne Hangx, Post Doctoral Researcher at the University of Utrecht
The research was conducted by an international consortium led by Cambridge University together with universities in Aachen (Germany) and Utrecht (Netherlands), the Jülich Centre for Neutron Science (Germany), Oak Ridge National Laboratory (USA), the British Geological Survey (UK) and Shell Global Solutions International (Netherlands). The Cambridge research into the CO2 reservoirs in Utah was funded by the Natural Environment Research Council (CRIUS consortium of Cambridge, Manchester and Leeds universities and the British Geological Survey) and the UK Department of Energy and Climate Change.





S. Chaudhuri
The amount of CO2 that can be stored in safe underground places remains a BIG question. The global economic impact of a CCS plan has to be thoroughly vetted—-science and economic feasibility must go together in such a massive plan. The science alone cannot provide a climate change mitigation solution. Readers may benefit from reading a novel concept outlined in http://www.climaterestore.com (one article has already been published by Robert C. Fry et al., 2015 in The International Journal of Climate Change: Impacts and Responses, vol. 8, 1-10, and the second article by the same authors is in press in the same journal). The material(s) gives an outline of how to accelerate a natural process to bring down rapidly the growth of CO2 in the atmosphere. The plan has the potential to create a new global economy that will benefit all countries of the world, providing jobs for the entire humanity, unlike the policies promoted by the developed countries at the Paris COP21 meeting.