According to the previous post, tetrahedral and octahedral sheets combine to form layers, and we can find two main types of clay structures: structure 1:1 (one tetrahedron sheet and one octahedral sheet) and 2:1 (two tetrahedral sheets and one octahedral sheet).
The basic structure of clays is this:
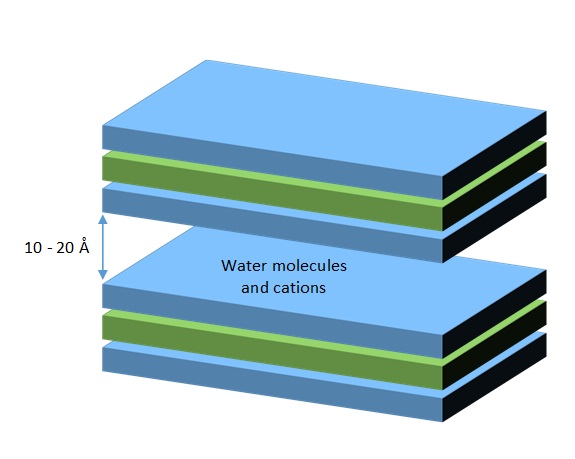
Substitutions between cations may occur in the tetrahedral and octahedral sheets, resulting in different charge deficits. These negative charges attract cations which are inserted between the layers, in the so-called interlayer space.
Depending on these substitutions, composition of the tetrahedral and octahedral sheets changes, and the resulting layer will have no charge, or will have a net negative charge. Depending on the amount of negative charge, the place where it occurs (the tetrahedral or octahedral sheet) and the type of cations, different mineral species may appear: kaolinite, serpentine, mica (muscovite, biotite, illite), smectite (montmorillonite), vermiculite, chlorite, sepiolite and vermiculite, mainly. Let’s have a look at them.
2-sheet minerals (1:1 structure)
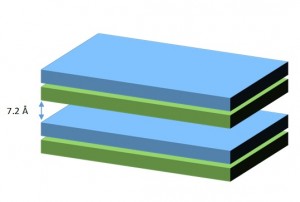
Some examples of 2-sheets minerals are kaolinite, dickite and nacrite (wich are polymorphs of Al2Si2O5(OH)4. Halloysite, a hydrated form of kaolinite, can be found in some Tropical soils.The structure of kaolinite is the following:
Layers of kaolinite are formed by a tetrahedral sheet of SiO44- on another sheet of AlOH66- octahedra, with shared vertices. The interlayer space is about 7.2 Å thick and non expandable due to strong hydrogen bonds. This union does not allow water molecules or ions to enter the structure. Particle size ranges from 0.2 to 2 µm and the effective surface area is limited (10 – 30 m2/g), as only external surfaces are available.

Kaolinite. Credit: M. Roe, Macaulay Institute. Click to see the original image and details at the Mineralogical Society website.

Halloysite (hollow tubes). Credit: Ömer Işik Ece, Istanbul Technical University. Click to see the original image and details at the Mineralogical Society website.

Kaolinite. Credit: Ray Frost, Macaulay Institute. Click to see the original image and details at the Mineralogical Society website.
In kaolinites, Si is never replaced. So, the elementary particle is electrically neutral and the cation exchange capacity (CEC) very low (1-10 cmol (+) / kg), which explains the low fertility of soils rich in kaolinite.
The name “kaolinite” is derived from Chinese Kao-Ling, a mountain from Jiangxi province (China) were this mineral was extracted.

Kaollinite-rich soil in Faro (Portugal). Credit: A. Jordán. Click to see the original image and details in Imaggeo.
3-sheet minerals (2:1 structure)
Smectites
Smectites are a group of clay minerals including pyrophyllite, montmorillonite, nontronite, beidellite and saponite. Montmorillonite is hydrated sodium calcium aluminium magnesium silicate hydroxide (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2·nH2O. Charge is very negative due to isomorphous substitutions of Si. Crystallization of montmorillonites is not stable, since the layers are bonded only by Van der Waals forces, weak O-O bonds and cation-O bonds, what makes the interlayer space very variable. This allows easy expansion of the crystal and the entry of water molecules and cations. On average, the spacing between adjacent layers is around 14.2 Å, but it may easily expand due to swelling in contact with water. During the dry season, water loss reduces the interlayer space, contraction of soil aggregates and development of cracks in the soil surface.As the internal surface of layers is available for interactions, the effective surface area is very high (up to 600 – 800 m2/g).

Montmorillonite. Credit: Emilia García-Romero, Universidad Complutense de Madrid. Click to see the original image and details at the Mineralogical Society website.

Smectite, nontronitic. Credit: Michael Velbel (Michigan State University) and William Barker, (University of Wisconsin-Madison). Click to see the original image and details at the Mineralogical Society website.

Summer cracks at the surface of a montmorillonite-rich soil from the Doñana Natural Park, Spain. Credit: A. Jordán. Click to see the original image and details at Imaggeo.
Micas
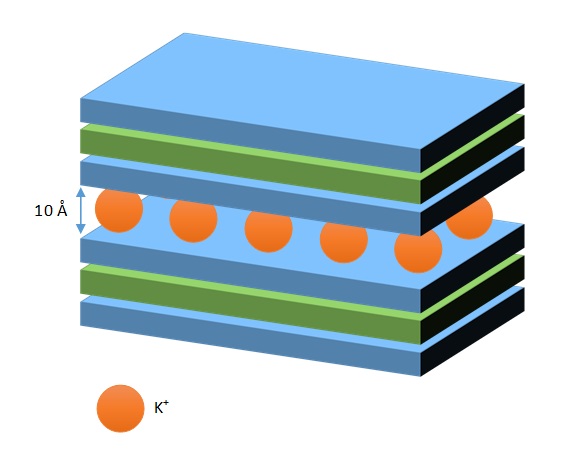
Micas are also three-layer minerals, but quite different from montmorillonites. Micas and illites (see below) are a group characterized the presence of trivalent cations in the octahedral sheet and potassium in the tetrahedral and octahedral seets. This allows them to have a greater CEC.
The unit cell is negatively charged, but is compensated by the entry of K+ ions. Mica crystallizes from magma, and isomorphous substitution of Al+3 for Si4+ is intense. Consequently, there is a highly net negative charge. K+ cations are strongly retained in the interlayer space, which can not expand or pick up other cations. CEC is low, and the interlayer spacing is constant (10 Å).
In addition to cations forming the crystal structure (Al, Si, Mg and Fe), there are also others between the layers, which confer give specific characteristics. These variations are the origin of muscovite (native of Moscow, Russia), biotite (in honour of the French physicist Jean-Baptiste Biot) and phlogopite (from a Greek word meaning “like fire”).
Illites
Illites are three-layer minerals derived from pyrophyllites, where the substitution of Si4+ by Al3+ is less intense, and the global negative net charge is smaller.
With less negative charge, K+ cations are not strongly bonded, so other cations of similar size (or smaller but hydrated cations) can enter the structure. Therefore, the space between layers is slightly variable (not as much as in montmorillonites), 10 Å on average. The effective surface area is smilar to montmorillonite’s, but CEC is smaller (20 – 40 cmol(+)/kg).

Fibrous illite. Credit: M. Roe, Macaulay Institute. Click to see the original image and details at the Mineralogical Society website.
Chlorites
The chlorite has many isomorphic substitutions in the tetrahedral and octahedral layers (Al3+ instead of Si4+ and Mg2+ instead of Al3+). The negative charge is compensated by a stable positively charged octahedral sheet of Mg, Fe and Al hydroxides sandwiched between the tetrahedral sheets. The expansion of the network is difficult, and the entry of water molecules and cations is limited. The effective surface area is small (70 – 100 m2/g).

Fe-rich chlorite from an alpine fissure. Credit: Michal Skiba, Institute of Geological Sciences, Jagiellonian University. Click to see the original image and details at the Mineralogical Society website.
Vermiculites
Vermiculites are not very frequent. These clay minerals are intermediate froms between chlorites and micas. Intense weathering removed some K+ cations, which are replaced by other hydrated cations. Expansion of the network is easy (10 – 15 Å), allowing the entry of water and cations replacing Mg2+. Net hegative charge is very high and CEC is . The effective surface area is 600 – 800 m2/g, similar to smectites.

Vermiculite. Credit: Steve Hillier and David Riley. Click to see the original image and details at the Mineralogical Society website. See video footage at the begining of the text, showing time lapse of hydrogen peroxide exfoliation process over 7 hours real time. Note bubbles in early stages when peroxide is still covering the particle, and relaxation as the process completes.
This post has been also published in gsoil.wordpress.com.