Post by Matt Herod, Waste and Decommissioning Project Officer for the Canadian Nuclear Safety Commission, and Adjunct Professor in Earth and Environmental Science at the University of Ottawa, in Ottawa, Canada.
_______________________________________________
I have always been a mineral and fossil collector. It was a hobby that stuck and blossomed into a career. I still collect minerals and fossils, although I’ve now added rocks from my field sites to the collection. One thing I should note is that for inanimate, immobile objects it is shocking how quickly rocks can colonize parts of a house, garage, basement, etc.
However, since my early years in geology a very large part of my day is concerned with water; my PhD was almost exclusively about water. Water is my focus and it is truly fascinating. So that got me thinking. Why don’t I collect water?
You may think water is all the same. Turn on the tap, it comes out, drink, wash, whatever. It’s just water. Well, you could not be more wrong. Water is different and changeable. Plus it fits in a small bottle. In short, the perfect collectible.
But maybe you’re not convinced to start collecting water just yet.
Water has types, an identity, just like people. You may be familiar with the notion of people’s personalities being Type A’s, B’s and C’s. Although the types of water are a little more nuanced. That said, so are the types of people.
Water is sorted into types based on its chemistry. The chemistry of water comes from the dissolved salts within it and the relative concentrations of those salts. The isotopic composition of water can also be used to identify its type. Some water types are classed based on their heritage. For example, water found in pore spaces deep underground is often called brine, the precursor to that brine is, or often was ancient ocean water.
Let me give you some examples of water with interesting identities. One thing I should mention is that many of the ways waters are typified only consider their dissolved salt concentration, however, when you factor pH, Eh, and isotopic variation of the many, many different isotopes the number of water types balloons exponentially. For example, a water with a pH of 6 and a total dissolved solids (TDS) concentration of 500 ppm can have isotopic ratios, age and origin totally different from another water with the same pH and TDS. Like I said, it gets complicated fast.
To start with an easy one:
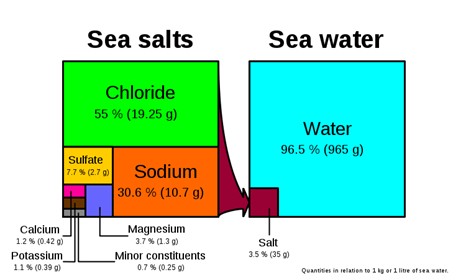
Seawater: Not only is it familiar, it is pretty important given that 97% of Earth’s water is this type and a significant percentage of Earth’s biomass lives in it. Seawater is about 3.5% saline and one of the most interesting features of the water is that it is pretty much everywhere and chemically very consistent. There are differences in the composition of seawater in the certain places around the world, for example, in restricted basins salinity can be higher or where fresh water enters the ocean in a river delta or estuary salinity is lower. Isotopically seawater is also interesting. Not because it has an unusual isotopic composition, but because seawater has been set as the standard to which all other water is compared. It is the zero point that stable isotopes in all other water is measured against.
Glacial Water: Of the 3% of Earth’s water left after the oceans, 69% is frozen in glaciers. To condense the characteristics of glacial water into one word I’d say clean. It just doesn’t have much in it. The reason for this is that glacial water started its days as precipitation, which is water that was evaporated from a water body and condensed. The evaporation process removes almost all of the dissolved solutes. Therefore, there just isn’t much stuff in glacial water besides the H and the O. That doesn’t mean glacial water is boring though. There is still a lot that if can tell us. For example, the variations in O isotopes can be used to reconstruct past temperatures and gases trapped in the ice can tell us about the composition of past atmospheres as well. The information we get from glacial water is different, but extremely valuable!

Brine: Do not drink a brine. You WILL regret it. I do not speak from experience, but frankly if almost 30% of the fluid is salt, it simply isn’t drinkable. Brines come in a lot of flavours, and technically if it has >5% salt it’s considered brine. However, I have encountered some brines that are over 30% saline. Of course, they were not drinkable as they were porewater in a sedimentary basin. However, there are some extremely salty bodies of water out there as well. Brines are interesting because they have so many stories to tell. There is history there, recorded by the solutes, gases and isotopic composition of the brine that explains how it became more than a simple water and transcended the label of water entirely to become much, much more…a fluid. Typically brines in nature have a history that involves salt dissolution leading to high concentrations of Na and Cl. However, other types may simply be evaporated seawater causing all of the dissolved ions to become more concentrated. For example, brines often have high concentrations of Ca, Br, I, Sr, etc, etc. Isotopes in brines also reveal a lot about their past and can distinguish if a brine is a glacial water that has dissolved salt, or is evaporated seawater or has a hydrothermal component. There is just always more that you can find out about brines.
High and low pH waters: pH plays a huge role in dictating the chemistry of water and the dissolved salts therein. Around the world there are naturally occurring waters that have incredibly high and low pH’s. The low pH waters, typically around 1-3 on the pH scale, occur in areas where natural acid rock drainage is happening. Acid rock drainage, aka. ARD, happens when sulphide minerals, often pyrite, oxidize releasing sulphuric acid leading to seeps with exceedingly low pH’s. On the other hand, high pH waters occur more rarely. Alkali springs occur when water comes in contact with hydroxide minerals, such as calcium hydroxide. Hydroxides form in dry, arid environments or where organics and limestone have been heated and burned such as areas with volcanic activity. One famous alkali spring is the Maqarin site in Jordan where water with pH’s from 11-13 occur!
Hydrothermal waters: HOT, HOT, HOT! These waters are absolutely loaded with interesting chemistry. Spewing out of hydrothermal vents on the seafloor at temperatures of up to hundreds of degrees Celsius with tons and tons of dissolved metals like copper, lead, gold, zinc, silver, etc. Furthermore, many of the world’s metal deposits are related to the movement of hydrothermal fluids within the crust. Hydrothermal fluids get their heat from the mantle or magma chambers within the crust. As they are heated they dissolve the rocks in contact with then leading to highly enriched solute concentrations and then when they discharge and cool, the solutes precipitate leading to black smokers, or mineral precipitates in fractures in the crust.
Young and old waters: My last Water Underground post discussed this in more detail. Suffice it to say when you start analyzing carbon-14, tritium, chlorine-36, iodine-129, krypton-85 and 81, etc. you can find waters ranging in age from just a few years to tens of millions to billions of years old. Each of these, is worthy of a spot on my shelf that is for sure. Excitingly, each of these waters has a story to tell about its origin and experiences over the years. Analyzing these isotopes and putting them in context with the geologic history of where the water is found can explain a lot about the regional hydrologic cycle and how water recharges, and discharges and how vulnerable the aquifer it is housed in is to contamination or over-pumping.
This is just the tip of the iceberg (see above). There are many ways water is sorted into types, often called “facies”, which are then plotted graphically. Here is a nice paper that compares some of the different ways of plotting water [1]. Read all the way to the end an you’ll be rewarded with a somewhat strange smiling face.
Anyway, hopefully I’ve convinced you to grab a bottle and collect a sample or two when you come across an interesting water!
Finally, my only piece of advice if you’re going to start a water collection…make sure the top is screwed on tight.
Reference
[1] Güler, Cüneyt, et al. “Evaluation of graphical and multivariate statistical methods for classification of water chemistry data.” Hydrogeology journal 10.4 (2002): 455-474.
_______________________________________________
 Matt Herod is a Waste and Decommissioning Project Officer for the Canadian Nuclear Safety Commission, and an Adjunct Professor in Earth and Environmental Science at the University of Ottawa, in Ottawa, Canada. To keep up to date with Matt, follow him on Twitter or on his own EGU blog GeoSphere!
Matt Herod is a Waste and Decommissioning Project Officer for the Canadian Nuclear Safety Commission, and an Adjunct Professor in Earth and Environmental Science at the University of Ottawa, in Ottawa, Canada. To keep up to date with Matt, follow him on Twitter or on his own EGU blog GeoSphere!