Post by Theodore Lim, assistant professor of Urban Affairs and Planning at Virginia Tech. He researches the socio-hydrology of green infrastructure planning and implementation. In order for people to care about something, to value it, they have to be able to see it and experience it. This point should not be taken lightly. So much about decision-making and policy-making depends on how much public ...[Read More]
Socio-hydrology meets Broadway: Can we survive drought if we stop using the toilet?
Post by Samuel Zipper, postdoctoral fellow at both McGill University and the University of Victoria, in Canada. You can follow Sam on Twitter at @ZipperSam. ___________________________________________________________ How can society best cope with water scarcity? With Cape Town on the verge of being the first major city to run out of water (a topic for a future post here on Water Underground), thi ...[Read More]
Happy birthday plate tectonics!
Post by Elco Luijendijk, a junior lecturer, and David Hindle, lecturer and head of geodynamic modelling, both at the Department of Structural Geology and Geodynamics at the University of Göttingen, in Germany. _______________________________________________ As we’ve firmly moved into 2018, we can say happy 50th birthday to one of the most revolutionary scientific theories of the last century: plat ...[Read More]
A cool new collectible: Water
Post by Matt Herod, Waste and Decommissioning Project Officer for the Canadian Nuclear Safety Commission, and Adjunct Professor in Earth and Environmental Science at the University of Ottawa, in Ottawa, Canada. _______________________________________________ I have always been a mineral and fossil collector. It was a hobby that stuck and blossomed into a career. I still collect minerals and fossil ...[Read More]
An alternate career path for Groundwater Science-Engineering PhDs
Post by Jim Roy, Research Scientist at Environment and Climate Change Canada. _______________________________________________ A recent editorial in Nature highlighted the relative scarcity of academic positions available to graduating PhD students (Many junior scientists need to take a hard look at their job prospects; 25 October, 2017). It notes that “it has been evident for years that internatio ...[Read More]
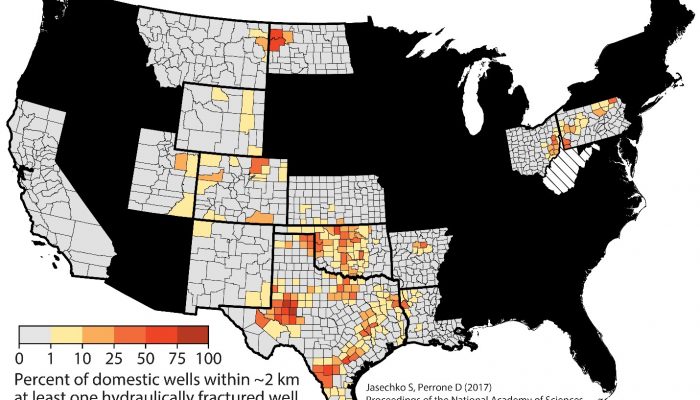
Hydraulic fracturing close to groundwater wells
Post by Scott Jasechko, Assistant Professor of Water Resources with the Bren School of Environmental Science & Management, at the University of California, Santa Barbara, and by Debra Perrone, non-resident Fellow at Water in the West and an Assistant Professor, also at the University of California, Santa Barbara, in the United States. _______________________________________________ In December ...[Read More]
How did our planet get its water?
Post by WaterUnderground contributors Elco Luijendijk and Stefan Peters from the University of Göttingen, in Germany. After my first ever scientific presentation, someone in the audience asked a question that caught me off guard: “Where does the groundwater come from?”. “Ehm, from rainfall”, I answered. The answer seemed obvious at the time. However, we did not realize at the time that this is a ...[Read More]
The great American groundwater road trip: Interstate 80 over the Ogallala Aquifer
Authored by: Sam Zipper – Postdoctoral Researcher in the Department of Civil & Environmental Engineering at the University of Wisconsin-Madison In late July, my wife and I loaded the dog into the car, cranked up the water-related tunes, and drove over a few million cubic meters of water. No, we haven’t traded in our sedan for an amphibious vehicle – rather, we were driving west, a ...[Read More]
FloPy: A Python interface for MODFLOW that kicks tail!
Authored by: Kevin Befus – Assistant professor, Department of Civil and Architectural Engineering at the University of Wyoming Groundwater modeling is getting better. Models are becoming more sophisticated with simpler interfaces to add, extract, and process the data. So, at first appearances, the U.S. Geological Survey’s (USGS) recent release of a Python module named FloPy for preparing, ru ...[Read More]
It’s out of the park! Comparing some of the water threats in America…
This is cool! a video is from Owen Miles’ watermurica blog