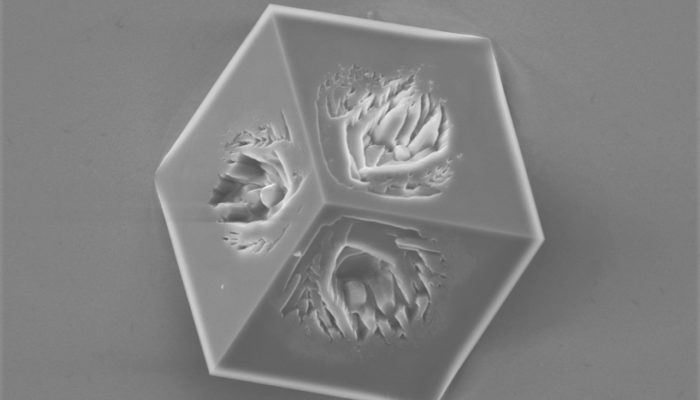
This imperfect cube of calcite was formed on the gold surface during the electrochemically assisted scaling process. Once negative electrochemical potential is applied to the gold surface, the oxygen dissolved in water undergoes reduction, yielding hydroxide anions. These anions accumulate at the gold-solution interface, forming a high pH layer. As calcite becomes less soluble with the increasing pH, it can precipitate on gold even from undersaturated solution. Like this wannabe cubic calcite crystal.
Photo by Joanna Dziadkowiec shared on imaggeo.egu.eu.
Imaggeo is the EGU’s online open access geosciences image repository. All geoscientists (and others) can submit their photographs and videos to this repository and, since it is open access, these images can be used for free by scientists for their presentations or publications, by educators and the general public, and some images can even be used freely for commercial purposes. Photographers also retain full rights of use, as Imaggeo images are licensed and distributed by the EGU under a Creative Commons licence. Submit your photos at http://imaggeo.egu.eu/upload/.




