By Penny Wieser (PhD student at the University of Cambridge)
Clues into the inner workings of volcanoes can be gleamed from material which is erupted at the surface, or that which solidified at depth in the crust. Just before eruption, three main phases are present: a gas phase (containing water, carbon dioxide, sulphur, chlorine etc), a liquid melt phase (the magma), and a solid phase (consisting of lots of different kinds of crystals).
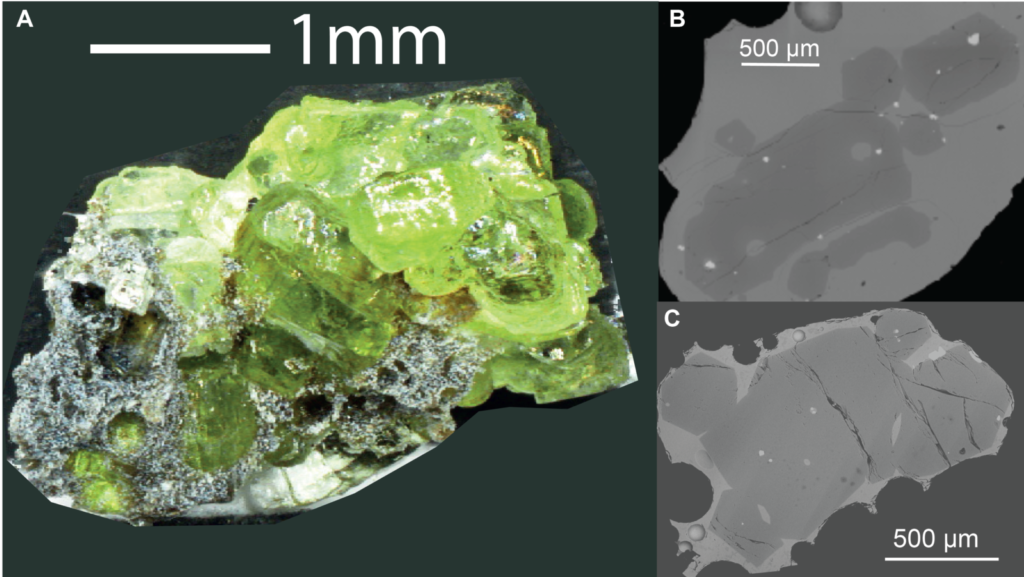
Olivine aggregate consisting of at least 17 separate crystals (taking with a normal camera). B-C) Backscatter electron images taken using an SEM showing attached olivine crystals (dark grey) surrounded by volcanic glass (solidified melt; lighter grey).
Petrologists spend their lives studying these crystals, and use them to determine how deep the magmas are stored in the crust, how long the magmas are stored, and how fast the magmas rise to the surface. However, many of the characteristics of erupted crystals are not fully understood, but may provide crucial insights into processes happening at depth within volcanoes. One example is individual crystals attaching together to form aggregates (also known as clusters, clots, or glomerocrysts). Despite these being really common in volcanoes, it’s still not exactly clear how they form. Previously it has been suggested that aggregates form during growth processes such as heterogeneous nucleation (where a new crystal nucleates on the surface of an existing crystal), dendritic growth (which occurs when crystals grow extremely fast, forming finger-like dendrites (this comes from the greek for ‘tree’)), or twinning (where two parts of a crystal grow together, with a specific orientation relative to one another). Or, crystals may “swim” together as they settle through a liquid magma column (through a process known as synneusis), or land on top of one another after they settle to the solid base of the magma chamber. We wanted to have a look at these different possibilities in some more detail, we did this using a technique called electron backscatter diffraction (EBSD).
We took olivine crystals erupted at Kīlauea Volcano (Hawai’i), and chromite crystals from the Bushveld Complex of South Africa, and prepared flat, very well polished slices. These samples were then placed in the Scanning Electron Microscope (similar to a regular microscope, but with electrons instead of light). An electron beam was focused on the sample; electrons which enter the sample are diffracted by planes of atoms in the olivine and chromite crystals, and leave the sample as “backscattered electrons” (these are collected by an EBSD detector). Following a series of data processing steps, these measurements can be used to determine the orientation of the crystal lattice. This provides a quick, quantitative way to determine the relative orientations of neighbouring crystals within aggregates. These observations can then be compared to the orientations expected from the various different hypotheses.
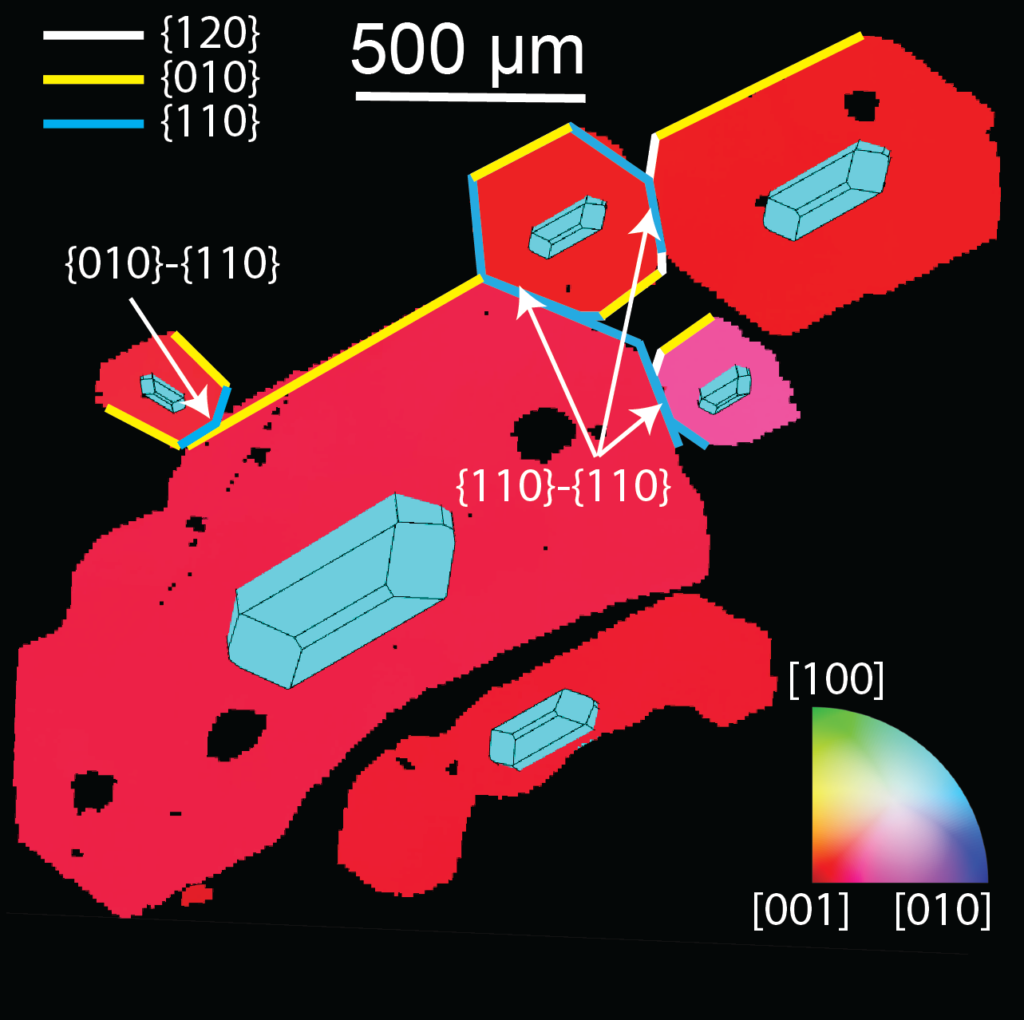
EBSD-map, color coded according to the orientation of the separate crystals. The fact that all 6 attached crystals show similar colors indicates that their orientations are similar.
Crystals are lazy; they will grow in the way that uses the least energy. During crystal growth, any mismatches in the direction of the new part of the crystal lattice with respect to the existing lattice is energetically exhausting. So if a new crystal wishes to grow on the surface of an existing crystal, it should mimic the orientation of the existing crystal to conserve energy. Similarly, twinning only produces a finite number of possible geometries, called “twin laws” (1 in chromites, 3 in olivines). In contrast, physical aggregation processes can result in a much broader range of attachment geometries. The settling of crystals onto the floor of a magma chamber where they land on other crystals produces totally random aggregate geometries (analogous to a very bad tetris player). The swimming together of crystals in the liquid part of the magma chamber results in certain crystal faces sticking together (which can be predicted from observations of the way that crystals orientate themselves as they fall).
Olivine and chromite aggregates show too broad a range of attachment geometries to have formed during crystal growth. Instead, we observe that large numbers of olivine crystals are attached along the crystal faces predicted from settling. So, just before eruption, olivines in in the liquid part of the volcanic plumbing system at Kīlauea Volcano must “swim together” as they settle. Chromite aggregates from the Bushveld complex show completely random orientations. This is best explained by the settling of individual crystals onto a firm substrate at the base of the chamber, where they randomly land on other chromite crystals. The fact that crystals in liquid magma are present as aggregates in some volcanic systems (e.g. Kīlauea), but individual crystals in others (e.g. Bushveld) has implications for calculations of the rates of crystal settling. This, in turn, affects the viscosity of erupted lavas (as slower settling rates means more crystals, and “stickier” magmas), and is important to understand the formation of economic deposits of platinum group elements (which are concentrated within settled piles of crystals within the Bushveld Complex).
Want to know more? Read all about it here!
 Penny Wieser is a PhD student in volcanology at the University of Cambridge, UK. She uses a variety of analytical techniques to interrogate erupted crystal cargoes and their melt inclusion records to gain insights into the pre-eruptive storage of magma beneath Kīlauea Volcano, Hawai’i.
Penny Wieser is a PhD student in volcanology at the University of Cambridge, UK. She uses a variety of analytical techniques to interrogate erupted crystal cargoes and their melt inclusion records to gain insights into the pre-eruptive storage of magma beneath Kīlauea Volcano, Hawai’i.

Pingback: Geochemistry, Mineralogy, Petrology & Volcanology | Tuesday EGU GMPV daily digest