Dear all BG members, welcome to EGU 2018! Remember our annual BG Division meeting that will be held on Thursday, April 12, 12:15-13:15 in Room L2. Enclosed the Agenda. This year, the Vladimir Ivanovich Vernadsky Medal of our Division is awarded to Antje Boetius. All invited at the Medal Lecture on Tuesday, April 10, 14:00–15:00 in Room C See you all this evening for the ice breaker at the Opening ...[Read More]
abstract submission
We are sorry to hear that people are experiencing difficulties submitting their last-minute #EGU18 abstracts & paying the APCs. If this is the case for you, you will be able to submit your abstract later today
Understanding the role of microbes in cold seep habitats
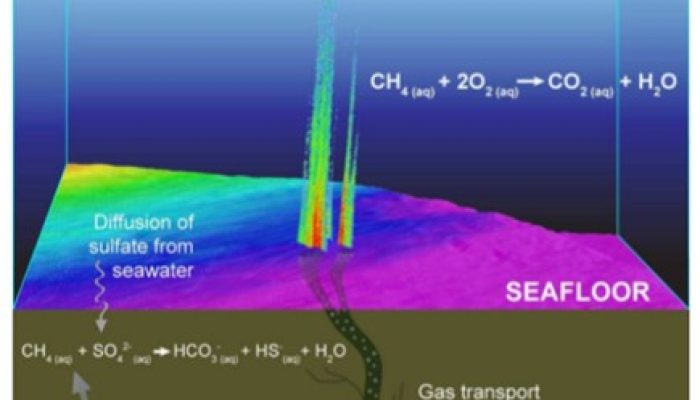
A cold seep is an area of the ocean seafloor where hydrogen sulfide, methane and other hydrocarbon-rich fluid seepage occurs. These parts of the ocean floor still remain a large mystery for scientists, in particular for the occurence of hydrothermal vents. In between these vents, microbes live that play a role in the local and global carbon budget. However their exact role remains largerly unknown ...[Read More]
Identification of past methane emission altering the foraminiferal tests by secondary overgrowth of calcium carbonate.
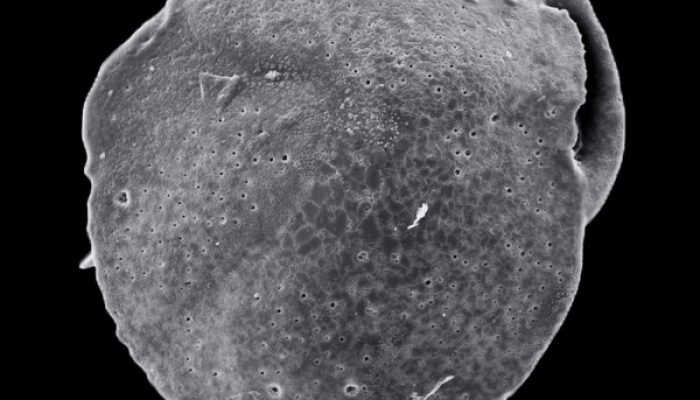
Ever heard about foraminifera? These tiny benthic (living at the seafloor) marine organisms are common in oceans across the globe and can be used to accurately give relative dates to sedimentary rocks. But we can also use them to identify past methane emissions from the seabed by studing their test or shell! The measurements were done on foraminifera called Cassidulina neoteretis , which is a ty ...[Read More]
Investigation of methane emissions in marine systems
Ever wondered how we can measure methane emssions from the seafloor ? And ever wanted to steer a mini submarine remotely operating vehicle (ROV)? Well here´s your chance! Have look at this blog post on analyzing methane emissions using ROVs and you´re ready to embark! The goal is to determine when the gas leak started and how the fluid flow systems work. With our research, we can contribut ...[Read More]
What´s in your fieldbag? Part 1: measuring freshwater carbon fluxes in the Artic
This bag belongs to Joshua Dean, Postdoc, Vrije Universiteit Amsterdam Field Work location Far Eastern Siberian Arctic: Kytalyk Nature Reserve. Duration of field work 2 weeks plus 3 days travel either side. Items in the bag Detecto Pak-Infrared (DP-IR) gas analyser [borrowed from colleagues, protect at all costs] EGM4 CO2 gas analyser [borrowed from another department, protect at all costs] water ...[Read More]
Coffee break biogeosciences – climate change affects mountain plant’s sex ratios

As climate change progresses, widespread changes in phenotypes in many plant populations are bing observed by scientists around the world. For instance in alpine areas, dominant plant species on lower altitude are shifting towards higher altitude as they adapt to increasing temperatures, thereby competing with high-altitude native plant species. In a recent study by Petry et al. (2016) it was show ...[Read More]
Keeping a lookout at the edge of the world

Few places in the world conjure up images of remoteness and harshness like Far Eastern Siberia. Yet, it’s places like these where our science is needed most. Arctic soils hold vast amount of carbon, protected in thick layers of permafrost, but these stores are becoming more and more vulnerable as temperatures in the Arctic warm, and are set to warm faster than anywhere else on the planet. R ...[Read More]
Coffee break biogeosciences – New coral reef at Amazon river mouth discovered
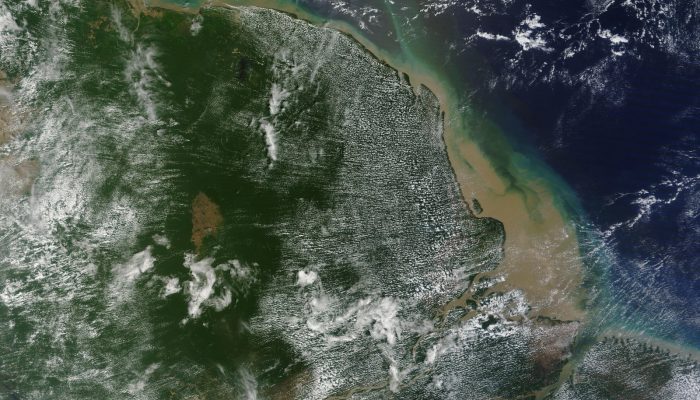
At the Amazon river mouth, a huge 9,300 sq km coral reef system has been found below the muddy waters off the mouth of the river Amazon. As corals mostly thrive in clear, sunlit, salt water, and the waters near the mouth of the Amazon are some of the muddiest in the world, the discovery of this almost 2000 km long reef leaves scientists puzzled about the potential extent of coral reefs worldwide. ...[Read More]
General assembly 2016: our session picks
Whether you’re an experienced attendee of the EGU GA, or this is your first time there are always things to look out for, it is always worth attending your division’s division meeting, one of the great debates, and keep an eye out for short courses which may be of interest to you. Here are the recommendations from the Biogeosciences Blogging team: The BG division meeting is Thursday from 12:15-13: ...[Read More]



