The March 2011 earthquake, 130 km off the coast of Japan, resulted in a 10-40 m high tsunami inundating Japan’s Pacific coast and caused the release of radionuclides from Fukushima Daiichi Nuclear Power Plant (NPP). The demise of three of the reactors was widely covered in the media, with worldwide coverage of the potential effects of radiation release both close to the plant and further afield.
Following the accident, significant quantities of radionuclides were released into the environment. Those of particular concern included 134Cs, 137Cs and 131I as large amounts of these isotopes are produced during nuclear fission and they both have high gamma ray energy. Radioiodine can enter the human body through food and drinking water, where it accumulates in the thyroid, creating an irradiation risk. However, the time required for 131I to decay is on the order of 8 days, so the irradiation risk rapidly decreases with time.
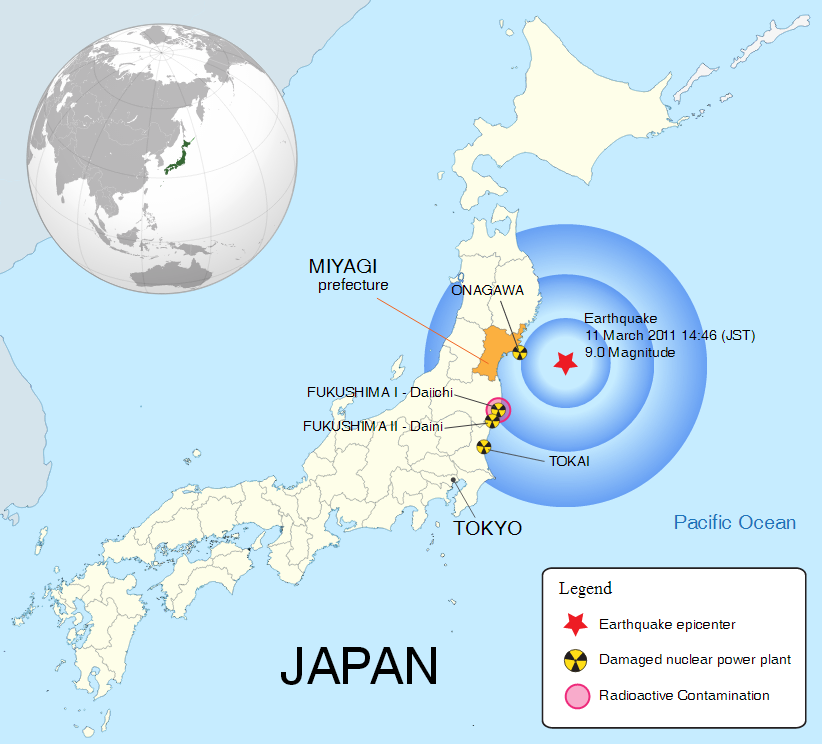
The location and impact of the March 2011 earthquake in Japan (click for larger). (Credit: Wikimedia Commons users W. Rebel and Jon C.)
This iodine isotope, however, has a much longer lifetime (it has a half-life of 1.57 x 107 years). This means that oceanographers can trace the path of the more harmful 131I retrospectively, based on where they find 129I (its decay product). There’s one caveat: the level of 129I in the environment is already elevated beyond natural concentrations. Release of 129I during nuclear fuel reprocessing has caused 129I to accumulate in both soils and seawater over time.
Consequently, knowing the background level of 129I is crucial to knowing what effect the Fukushima NPP accident had on the local environment. Thanks to a little routine water sampling, water samples from the northwest Pacific were taken before the plant went into operation – phew! So, we know that the concentration of 129I before the Fukushima NPP accident: it was on the order of 1-2 x 10 x 107 atoms per litre – what happened after it?
Takashi Suzuki and his team from Japan’s Atomic Energy Agency took a closer look at the 129I in water samples at the surface and at depth following the event and found elevated 129I throughout the water column, particularly in the surface mixed layer.
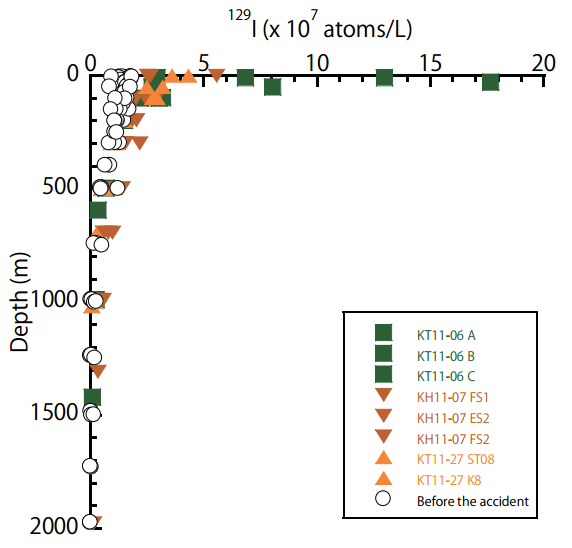
Depth profiles of 129I before and after the Fukushima NPP accident. Green, brown and orange points were from late April, early June and late October 2011, respectively. (Credit: Suzuki et al. 2013)
129I ranged from 1-90 x 107 atoms per litre after the accident, the highest measurement being a whopping 73 times larger than pre-accident levels! That certainly sounds like a lot, but what does it mean for the consumers of local seafood?
Using the concentration of 129I in the water column, the accumulation of radioactive iodine in marine animals and the amount of seafood consumed by average Japanese individual, Suzuki found that the risk to human health was negligible (6.7-550 x 10-11 Sv per year), some 100 million times lower than the annual does limit (1.0 x 10-3 Sv per year)! Good news for all concerned.
By Sara Mynott, EGU Communications Officer
These findings are published as part of a series on the impacts of Fukushima’s NPP discharges on the ocean. Published in Biogeosciences, this series brings together research on the dispersal pathways, transport processes and deposition of radionuclides following the accident, which you can access here.
References:
Aoyama, M., Uematsu, M., Tsumune, D., and Hamajima, Y.: Surface pathway of radioactive plume of TEPCO Fukushima NPP1 released 134Cs and 137Cs, Biogeosciences, 10, 3067-3078, 2013.
Suzuki, T., Otosaka, S., Kuwabara, J., Kawamura, H., and Kobayashi, T.: Iodine-129 concentration in seawater near Fukushima before and after the accident at the Fukushima Daiichi Nuclear Power Plant, Biogeosciences, 10, 3839-3847, 2013.

Bruce Napier
I-129 is NOT a decay product of I-131.
I-129 is produced separately by fission.
I-131 decays eventually to stable Xe-131.
You immediately lose credibility to the reader if you say I-131 decays to I-129.
Laura Roberts-Artal
Thank you for pointing this out. We have now updated the post.
Pingback: Lecturas de domingo (3) | Ciencias y cosas
Pingback: Following Fukushima: What happened to the iodin...