The Rare Earth Elements, or REEs, are really important. This is a group of elements including neodymium (used to create strong magnets), cerium (used in catalytic converters), lanthanum (used in electric car batteries), lutetium (used in oil refinery), with the uses of REEs increasing continuously.
At the moment, the majority of the world’s supply of REEs comes from a single deposit in Inner Mongolia (China) called Bayan Obo (in Mongolian, bayan means rich, an obo is a sacred stone pile). When one country supplies most of such an important resource, the rest of the world gets nervous, especially given current and (probable) future geopolitical tensions. Because of this, many countries are scrambling to find their own REE deposits.
In many of the REE deposits, the elements are held in minerals that are often considered to be somewhat exotic, including bastnaesite, apatite, monazite and allanite. But, in a paper in the journal Geology, Soenke Brandt and a team from the Friedrich-Alexander University of Erlangen-Nürnberg propose that olivine, a relatively common mineral, could be a major host of the REEs, at least in some special cases, including in Vergenoeg, South Africa.
Olivine is a common mineral in the Earth’s upper mantle, but it is generally considered to be a pretty poor host of trace elements, especially the REEs. In many cases, even measuring all of the REEs in olivine using our current analytical capabilities is either difficult or impossible, with concentrations in the order of parts-per-billion being not-too-uncommon in natural olivine*** (one part per billion means one gram in one thousand metric tonnes). Such low concentrations would mean that the cost of mining the rocks and processing them to remove the REEs would be far higher than the value of the REEs that would be extracted.
This is not the case in Vergenoeg. According to the study, olivine crystals from this deposit contain up to 6000 times higher concentration of REEs than chondrite (this is a type of meteorite that we often use for normalising geochemical data). Basically, it’s a lot – these are the highest values ever reported for some of the REEs in natural olivine.
Particularly interesting is the way in which these REEs are held in the olivine crystal structure. Generally, understanding how any element incorporates into a crystal is essentially a matter of charge-balance, arithmetic and ionic radius. Elements form ions when electrons are added or taken away from the atoms in their raw, untouched state, and therefore each ion has a positive or negative charge (positive = fewer electrons, negative = more electrons). If we want to put an ion with a given charge into a mineral, we have to remove ions with the same charge, or add in ions with the opposite charge. To put an ion with a charge of +2 into a crystal, we have to either remove an ion with +2 charge, or add in another ion with a charge of -2, so the end result equals zero.
The ionic radius is also important – we can simply think about this as the size of an ion, but there are a bunch of subtleties and complications (like, the radius of the hydrogen ion can be negative 🙈). Big ions want to swap places with other big ions, small ions want to replace small ions. Big ions can’t fit in small sites, and small ions often aren’t happy in big sites, they get kind of lonely.
The particular type of olivine (olivine is a broad term for a series of different mineral compositions) is fayalite, with the chemical formula Fe2SiO4. The Fe is iron, with a charge of +2 (Fe2+), the Si is silicon, with +4 charge (Si4+), and the O is oxygen, charge -2 (O2-). So in its normal state, fayalite is 2xFe2+ + 1xSi4+ + 4xO2-, so 2×2+4-4×2=0. The total charge is zero – that’s charge balance in action.
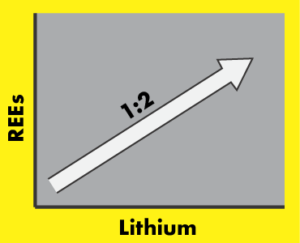
The ionic radii of at least some of the REEs is about the same as Fe2+. So taking iron out of the crystal, and putting REEs in, should be an easy swap. But, the charge is different – most REEs have a charge of +3, but this iron is +2. That means, to maintain charge balance, we have to get creative. The most commonly cited method of sticking a REE into olivine is to pull out three Fe2+, and put in just two REE3+ , leaving a hole (a vacancy) where one of the Fe2+ used to be (3xFe2+ out, 2xREE3+ in, 2×3-3×2=0). Another method is to pull out one Fe2+, and then one Si4+, and stick a REE3+ where the Fe2+ used to be, and an Al3+ (aluminium with a charge of +3) where the Si4+ used to be (1 Fe2+ and 1 Si4+ out, 1 REE3+ and 1Al3+ in, 2+4-3-3=0). In the Brandt study, they propose that the addition of REE3+ could be associated with Li+ (lithium with a charge of +1). Two Fe2+ come out, and one of the original sites is filled with Li+, and the other with REE3+ (2xFe2+ out, 1 REE3+ and 1Li+ in, 2×2-3-1=0).
If this is the case, then the number of atoms of lithium should equal the number of atoms of the REEs. In fact, Brandt measured about twice the concentration of lithium than REEs, but the correlation between the concentrations is pretty convincing. There is something else going on that isn’t really clear, but their evidence for a REE3+ plus Li+ incorporation mechanism looks solid. What is cool is that while this has been seen in experiments, it has not yet been shown so clearly as this in natural olivine. Maybe ‘cool’ is the wrong word, but a lot of petrologists and mineralogists (like me) spend much of their time thinking about this kind of thing…
Despite the complexities and interesting scientific avenues that this opens up, a major take-away is that olivine might not be so barren after all, and could be a potential REE resource for the future. Whether this kind of deposit is economical or not, meaning, whether the cost of mining and processing is less than the value of the REEs on the open market, is unclear, but maybe this study will encourage other geologists to look to olivine for future deposits.
***there are plenty of experimental studies where much higher concentrations of REE have been observed in olivine, but these are generally in quite REE-enriched systems.