
In this blog I’m going to talk a bit about one of my favourite rocks – the strangest rock you’ve probably never heard of – listvenite. Listvenite (sometimes spelt listwanite or listwaenite) is the product of a chemical reaction between peridotite and carbon dioxide, and it is truly strange! I first came across listvenites working on the Semail Ophiolite, Oman, during my PhD.
The Semail Ophiolite is a full section of oceanic crust and uppermost mantle which was emplaced as a sliver on the edge of the Arabian continental plate about 96 million years ago, during what can loosely be described as some kind of glitch in a subduction zone*. In places, mantle rocks in the lowest stratigraphic section of the ophiolite have been transformed to listvenites by reaction with CO2 apparently coincident with subduction and the emplacement of the ophiolite [1]. In 2017 the listvenites and the major subduction zone fault underlying them were sampled by diamond wireline coring during the Oman Drilling Project giving us amazing new samples which preserved a host of features which had weathered away in the arid environment at the surface [2].
(*one possible model for formation of the Oman Ophiolite is that the thinned edge of the Arabian continent got partially subducted, subduction stalled and finally the buoyant continental crust rebounded with the oceanic crust and mantle on top of it which now form the ophiolite)

Pillow lavas from the top of the oceanic crust sequence in the Semail Ophiolite, Oman. These are known as the Geotimes Pillow Lavas after a photo of them featured on the cover of Geotimes magazine in 1975. (photo courtesy of Georoamer, CC BY-SA 4.0)
Four of the strangest and best things about listvenites
These rocks are fascinating and beautiful for a whole bunch of reasons but here I’m just going to summarise four of the strangest things about them and why I think they are so interesting.
1. They are ultramafic rocks that are full of quartz!
Listvenites are mineralogically very weird. If the title of this section doesn’t immediately set of strangeness alarms for you then I’ll try to explain. We generally think of igneous rocks in simple terms as being on a spectrum between mafic and felsic, or in other words between being silica poor and silica rich. We see this is the difference in composition between a basalt (mafic, magnesium rich, silica poor) and a granite (felsic, silica rich, magnesium poor). Peridotites – the precursor rocks that listvenites form from – are right at the silica poor end of this spectrum; they are composed almost entirely of olivine and are so mafic that they are termed ultramafic rocks.
So why do we not expect quartz in an ultramafic rock? This essentially comes down to what minerals are in equilibrium with each other. In simple terms, we don’t expect an ultramafic rock to contain quartz because the silica-poor minerals like olivine which make up the bulk of the rock are thermodynamically unstable in the presence of quartz. Thermodynamically unstable is really just another way of saying we would get a chemical reaction and this is indeed what we see in cases where an olivine rich mafic magma has picked up quartz from another rock – quartz and olivine will react to form pyroxene. Put another way – we would never expect a magma (or any other material for that matter) to crystallise both olivine and quartz simultaneously. We get a good sense of this intuitively just looking at the typical crystallisation order of minerals from magmas (known as Bowen’s reaction series).
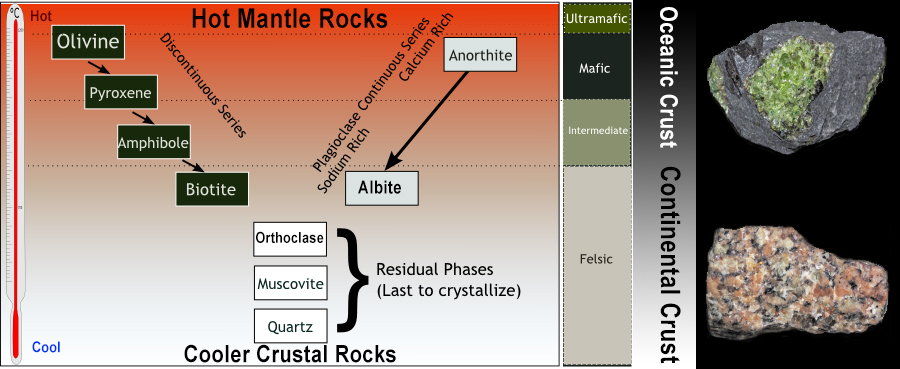
Diagram of Bowen’s reaction series depicting the typical crystallisation sequence of igneous magmas from ultramafic minerals like olivine to felsic minerals like quartz. Shown at the right are examples of ultramafic rocks (green perdotite xenolith in a basalt) and felsic rocks (granite). (Image by Benjamin J. Burger, CC BY-SA 4.0)
So maybe while it’s being transformed into listvenite we’ve added loads of silica to our poor peridotite and that’s why there’s quartz? Well, here’s the interesting thing, for most elements (though not all as we’ll see later) the average composition of the listvenites is identical to unaltered peridotites from the same unit. So our rock looks chemically like a normal piece of the mantle and hasn’t had any additional silica added but, despite this, a major component of it is quartz [3].
The real reason behind this and all the other mineralogical weirdnesses of listvenites is the elephant in the room: carbon dioxide. As I mentioned in the introduction, listvenites are carbonated peridotites and it is the reaction of silicate minerals with all that CO2 which gives them such a strange and unusual mineralogy. To fully understand what’s going on we should look at what else makes up the bulk of our rock.
Alongside quartz, the majority of the listvenite rock is made up of dolomite (calcium-magnesium carbonate, CaMg(CO3)2) and it’s lesser-seen cousin magnesite (magnesium carbonate, MgCO3). This makes clear that what has happened is a wholescale reorganisation of the rock. What was once a silicate, formed of various minerals like olivine (Mg2SiO4) or clinopyroxene (MgCaSi2O6) consisting of metals and silica has been transformed into a carbonate where those same metals (mostly Mg, Fe and Ca) are now in carbonate minerals magnesite, dolomite or sometimes calcite. And the reason we have a load of quartz is simply that all that silicon and oxygen from our silicate minerals has to go somewhere! (If you’re wondering why we don’t instead get silicon carbonate then here’s a good place to start: [4])
2. They are often bright green!
The mineralogical weirdness of listvenites doesn’t stop at quartz. You also have counter-intuitive combinations like (oxidised) haematite and (reduced) graphite happily coexisting. Added to that, the complete transformation from a silicate-based rock to one dominated by carbonate leaves a few other elements, much like silicon, in a bit of fix with nowhere to go This leads to some weird and wonderful minerals. The prettiest of these is definitely fuchsite – a rare chromium rich mica. This forms in several places in the listvenites and takes up chromium liberated from chrome-spinel which is an accessory phase in the peridotite precursor rocks.

Photos of listvenite core samples of with abundant fuchsite (chromium mica) showing the range of bright green colours and textures. None of the photos have been colour adjusted or enhanced in any way. (For core images and loads more open data see [2])
The fuchsite seems to form only in areas of the rock that have elevated aluminium and potassium and so it seems to be a sort of marker for some earlier metasomatic (i.e. infiltration of fluid or magma which chemically and mineralogically changes the rock) event [3]. It’s also noteworthy for having a beautiful green colour which makes many of the horizons where it is abundant look like beautiful peacock feathers!
3. They are full of arsenic!
I said earlier that the listvenites on average have the same composition as the peridotites they formed from. However as I hinted at, that’s not exactly true. The major elements (Mg, Si, Fe, Ca, etc) which make up most of the rock are indeed mostly indistinguishable, however there are several elements which show extremely elevated concentrations in the listvenites: one of these is every poisoner’s best friend, arsenic.
Now before you get worried, no-one is going to poison anyone with a listvenite. They contain, at most, about 200 parts per million (ppm) of arsenic by weight. However, arsenic is an extremely rare element; in the Earth’s mantle we think there is only about 0.008 to 0.05 ppm, meaning that in our listvenites it has been enriched by a factor of up to 25,000! Other elements are similarly enriched, including lithium, antimony and lead [3]. These are all what are known as fluid mobile elements which are easily leached from rocks and moved around by water or other fluids. Because of this, it seems very likely that the fluids responsible for carbonating the rocks were also responsible for the huge enrichments in fluid mobile trace elements we see.
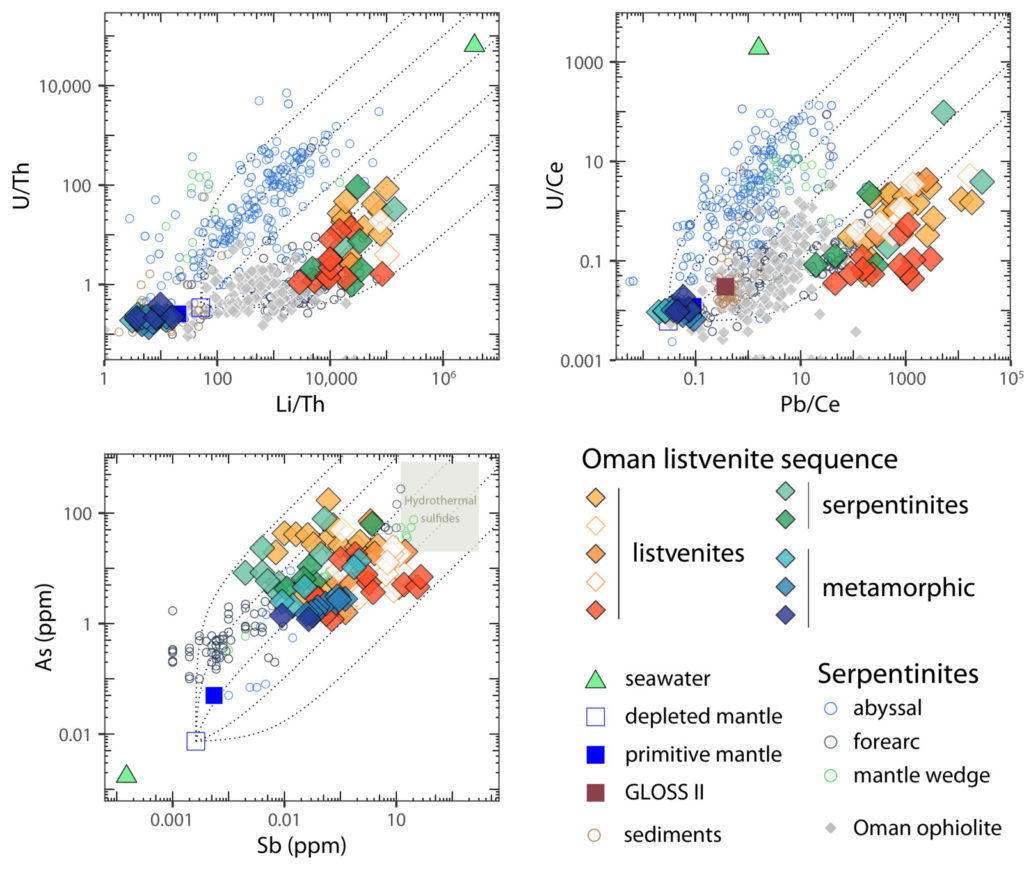
Graphs showing enrichment of fluid-mobile elements including arsenic, lithium, antimony and lead in listvenites (relative to elements that behave similarly during igneous processes). The pattern of enrichment is very similar to that seen in forearc serpentinites suggesting listvenites may have a similarly important control on the behaviour of these elements in subduction zones. (Listvenite data from [3], forearc, and abyssal serpentinite data from [5])
What is particularly interesting about this is that the pattern of enrichment of fluid mobile elements is really similar to, but even more extreme than, that seen in serpentinites from the forearcs of subduction zones [5]. The Oman listvenites are thought to have formed in a very similar environment and are directly underlain by what is interpreted as the plate boundary of a fossil subduction zone. The enrichment of fluid mobile elements in subduction zone serpentinites is thought to be a really important control, modulating the passage of these elements into the mantle and arc volcanoes. The similarity of the geochemical patterns and setting of the Oman listvenites suggests they could play a similarly important role on the cycles of these elements from the surface and into the deep Earth.
4. They (might) lock up your CO2
The last and probably most important thing about listvenites is that they contain a lot of CO2, locked up in inert and highly stable minerals (particularly magnesite which is difficult to break down even with acid). This means they have got quite a bit of attention as a potential way to remove CO2 from the atmosphere and lock it away – so called carbon capture and storage (CCS) [6]. In that context it’s worth noting that the hill near where the Oman listvenites were cored is estimated to contain around a billion tons of CO2! There’s a lot of work to do to determine if the reaction rates, temperatures and CO2 concentrations required are practically achievable. However, if they can help to mitigate against the worst of anthropogenic climate change, then it’d be another great reason to get excited about the ever exciting ever surprising rock that is listvenite.
References
[1] Falk E. S. and Kelemen P. B. (2015) Geochemistry and petrology of listvenite in the Samail ophiolite, Sultanate of Oman: Complete carbonation of peridotite during ophiolite emplacement. Geochim. Cosmochim. Acta 160, 70–90. http://doi.org/10.1016/j.gca.2015.03.014
[2] Kelemen P. B., Teagle D. A. H., Matter J. M., Coggon J. A. and The Oman Drilling Project Science Team (2020) Proceedings of the Oman Drilling Project. Coll. Station. TX (International Ocean Discov. Program). http://doi.org/10.14379/OmanDP.proc.2020
[3] Godard M., Carter E. J., Decrausaz T., Lafay R., Bennett E., Kourim F., Obeso J. ‐C., Michibayashi K., Harris M., Coggon J. A., Teagle D. A. H. and Kelemen P. B. (2021) Geochemical Profiles Across the Listvenite‐Metamorphic Transition in the Basal Megathrust of the Semail Ophiolite: Results From Drilling at OmanDP Hole BT1B. J. Geophys. Res. Solid Earth 126. https://doi.org/10.1029/2021JB022733
[4] Santoro M., Gorelli F., Haines J., Cambon O., Levelut C. and Garbarino G. (2011) Silicon carbonate phase formed from carbon dioxide and silica under pressure. Proc. Natl. Acad. Sci. U. S. A. 108, 7689–7692. https://doi.org/10.1073/pnas.1019691108
[5] Peters D., Bretscher A., John T., Scambelluri M. and Pettke T. (2017) Fluid-mobile elements in serpentinites: Constraints on serpentinisation environments and element cycling in subduction zones. Chem. Geol. 466, 654–666. http://dx.doi.org/10.1016/j.chemgeo.2017.07.017
[6] Kelemen P. B., Matter J., Streit E. E., Rudge J. F., Curry W. B. and Blusztajn J. (2011) Rates and Mechanisms of Mineral Carbonation in Peridotite: Natural Processes and Recipes for Enhanced, in situ CO2 Capture and Storage. Annu. Rev. Earth Planet. Sci. 39, 545–576. https://doi.org/10.1146/annurev-earth-092010-152509


Karyn
Thank you! Was beach rockhounding and we found quite a bit of green stone. This blog is gonna help me narrow it down!