“Tsunami“ is a word that became world famous after the so-called Christmas tsunami in 2004, when enormous waves hit the shores around the Indian Ocean with disastrous consequences for countries such as Sri Lanka, Thailand, Somalia and many others. But did you know that tsunamis can be icy? An ice tsunami is one of the many names associated with ice shoves (or ivu, shore ice override, i ...[Read More]
Image of the Week — ice tsunamis !
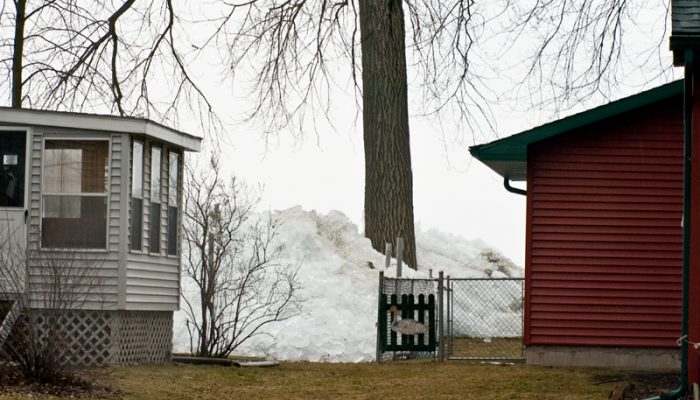
A pile-up ice shove at Brighton Beach, Winsconsin, in March 2009. [Credit: bigcityal via Wikimedia ]

