
The utility of logarithmic scales is nothing new to scientists – yet, sound interpretation of pH changes when comparing settings with different initial pH can be challenging. This blogpost highlights a manuscript by Fassbender et al. [1] that was recently submitted to the journal BG and is currently under open peer review.
The pH scale was first published by Søren Peter Lauritz Sørensen in 1909 [2], who worked on this in Copenhagen, Denmark. Concerns about the potential for misinterpretation of Sørensen’s pH scale were raised already early on [3]. Now, Fassbender et al.’s technical note focuses on the potential for misrepresenting the meaning of pH changes (see Fig. 1). They present three examples for why it would be important to “go beyond reporting changes in pH alone” when discussing changes in marine science pH scales.
For example, when assessing ocean pH changes, Fassbender et al. recommend to show results as Δ[H+], ΔpH, and when reporting pH data, to include the reference conditions along with the changes in order to avoid concealing or overemphasizing trends and patterns in ocean acidification. As surface ocean pH declines across the globe and ocean pH is considered an Essential Ocean Variable [4] and an Essential Climate Variable [5], understanding changes and variability in ocean pH is highly relevant.
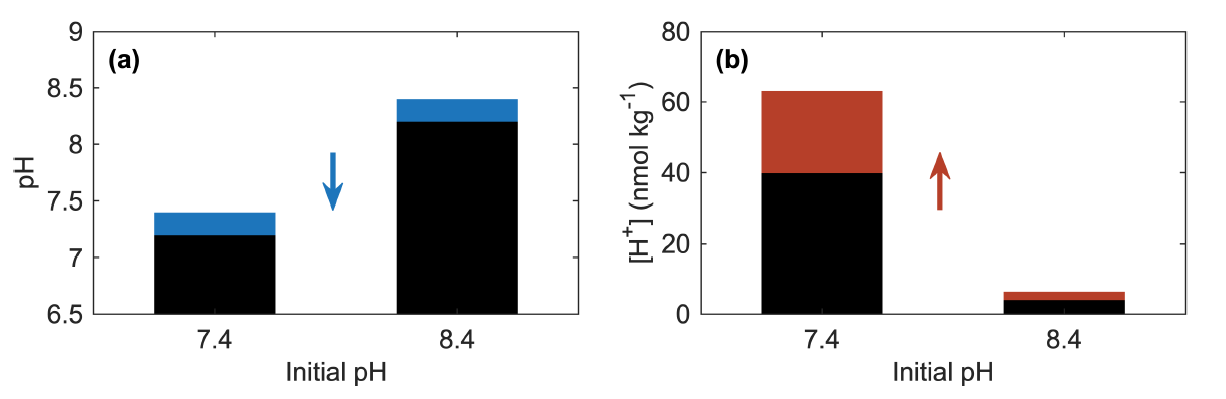
Figure 1. A 0.2 unit decrease in pH (blue portion of bars) equates to (b) a 58% increase in [H+] (red portion of bars) for both initial pH values of 7.4 and 8.4. The absolute change in [H+] depends on the initial conditions. (Fassbender et al. in review)
Q @Fassbender: What prompted you to write about the interpretation of ocean pH?
Only recently has the oceanographic community amassed the observational datasets required to characterize long-term pH trends in many ocean domains. As trend assessment is becoming more common, both in observations and numerical models, it seemed timely to make the argument for including [H+] when considering pH changes.
Q @Fassbender: Why submit to an open peer-review process?
This topic is relevant to a variety of oceanographic disciplines and analyses, so we selected the open peer-review process to enable consideration of community input, in addition to that of the formal reviewers, if it were to arise.
References:
[1] Fassbender, A. J., Orr, J. C., and Dickson, A. G.: Technical note: Interpreting pH changes, Biogeosciences Discuss. [preprint], https://doi.org/10.5194/bg-2020-348, in review, 2020. https://doi.org/10.5194/bg-2020-348, in review, 2020.
[2] Sørensen, S. P. L.: Enzymstudien II: Über die Messung und Bedeutung der Wasserstoffionen-konzentration bei biologischen Prozessen, Biochem. Zeit, 21, 131–200, doi:10.1007/BF02325444, 1909.
[3] Clark, W. M.: The determination of hydrogen ions, 2nd Edn., Williams and Wilkins Company, Baltimore, MD., 1922.
[4] GOOS: Essential Ocean Variables, available from: http://www.goosocean.org/index.php?option=com_content&view=article&id=14&Itemid=114 (accessed Dec 27, 2020).
[5] GCOS: The Global Observing System for climate: implementation needs. https://library.wmo.int/doc_num.php?explnum_id=3417, 2016.
[6] Carstensen, J., and Duarte, C. M.: Drivers of pH variability in coastal ecosystems. Environmental science & technology, 53(8), 4020-4029, 2019.
Blogpost written by Alexandra Rodler
