Post by Matt Herod Welcome to the first edition of groundwater speed dating. In today’s post I introduce you to a motley crew of isotopes and chemicals that hydrogeologists and geochemists use to date the age of groundwater. After meeting all of the contestants it will be up to you to pick your favourite and perhaps propose a second date. On your groundwater samples that is. Before I introdu ...[Read More]
Tracking the Fallout and Fate of Fukushima Iodine-129 in Rain and Groundwater
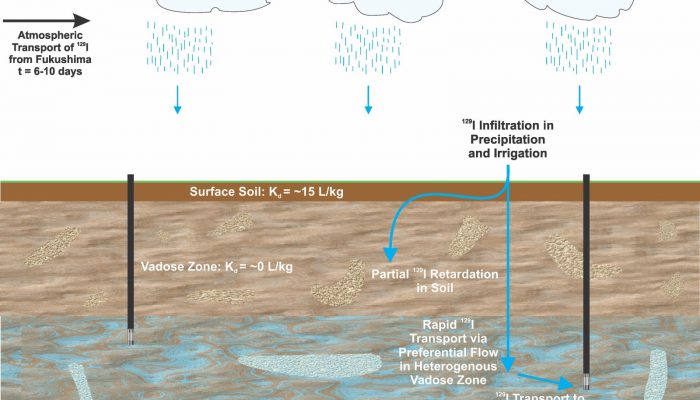
This post is written by Matt Herod, and reposted here with permission… A recently published paper (by myself and colleagues from uOttawa and Environment Canada) investigates the environmental fate of the long lived radioisotope of iodine, 129I, which was released by the Fukushima-Daichii Nuclear Accident (FDNA). Within 6 days of the FDNA 129I concentrations in Vancouver precipitation increas ...[Read More]