Sometimes our projects take stage and unexpected turns down pathways that we have no experience in whatsoever. My project on the input of Fukushima iodine-129 into groundwater has taken one of those turns. This is not a bad thing, but it is a time consuming one, as these deviations often are. However, instead of bemoaning my new lot in life as modeller of the unsaturated (vadose) zone, I thought I’d share a bit of what I’m doing. By the way, if any of you reading this have any experience modelling the vadose zone I’d love to talk in greater detail with you.
Modelling is an interesting field. The basic idea behind modelling is to try and make a computer program predict what is happening in reality by putting in a bunch of experimental or estimated parameters in and letting the model work through calculations that predict what would happen in nature. This is terrific since it is impossible for me to actually observe what is happening in the vadose zone of my field site. Sure I can do a bunch of experiments to try and empirically measure what is happening over time in the field. However, the cost and time is simply too prohibitive and there is no guarantee that I could even sample in such a way as to get good data back. This is the sort of circumstance where the model really comes into its own.
Models are great, but it is important to remember they do not provide the final answer. It doesn’t matter what is being modelled, the real world is orders of magnitude more complex than the model. Therefore, drawing conclusions based solely on the results of modelling is risky and certainly provides and incomplete picture of reality. Furthermore, models provide such pretty pictures and simple explanations for complex phenomena that it is easy/tempting to fall into the trap of over concluding from the model results. Therefore, when using a model to try to simulate reality is it very important to take the conclusions and results with a grain of salt whether you are the modeller, reviewer or reader.

A picture from my model showing 129I transport in grams. The height of the column is proportional to depth.
The beautiful column of blue and green above shows a very preliminary attempt by me to model the transport of iodine-129 through the vadose (unsaturated) zone of an aquifer over a period of time in 2011. The way the model works is I input a bunch of data (educated or even not so educated guesses) on the soil conditions such as porosity, hydraulic conductivity, moisture content and much more. Then I add in the amount of rain that fell on my study area that has a known concentration of iodine. I then let the model run and watch the 129I infiltrate into the ground. When it gets to the bottom that means it has reached the water table. I can then look through the dataset that the model produces and see the number of grams of 129I that have travelled through the vadose zone and how long it took for them to do so.

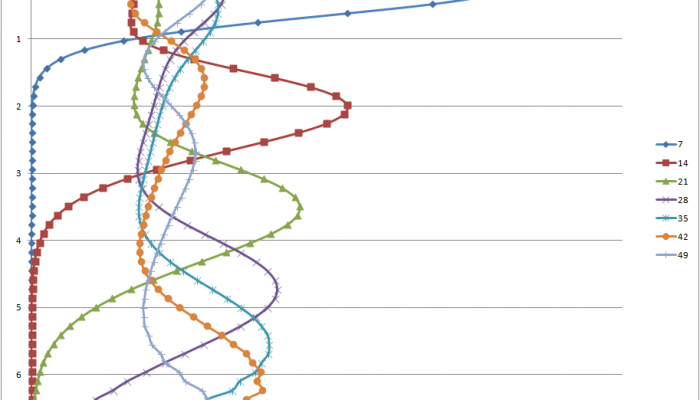
A graph of the model output showing concentration with depth. Each line represents a different snapshot in time. e.g. 7 represents 7 days.
The next step once the model produces a scarily large table of numbers is to try and make some sense of what happened. The blue-green thing is nice, but it doesn’t give a whole lot of information about what is happening over time at different depths. With this table I can then make graphs showing the movement of my iodine over time into the vadose zone. As you can see the “peak” migrates downwards pretty rapidly and within just over a month has moved 7 metres! Of course, this result makes us all think that iodine infiltrates into groundwater really rapidly. The actual truth is not nearly so black and white however. In fact, if I tweak the parameter that controls how iodine sticks to soil I can prevent it from ever getting past the ground surface. This is why all models should be taken with several grains of salt. The input control the outputs, however, a small change in the input can result in a radically different output. Which is the truth??? Only observation and more modelling can tell us!
[polldaddy poll=”7974309″]