Dear Readers!
Christmas is almost upon us and so at Four Degrees we decided to devote our next post in the ‘What’s Geology got to do with it?’ series to Christmas! Marion and I have selected varying aspects of the festive season from trees to biblical stories and common Christmas presents, and linked them to geology (some tenuous, some not so tenuous…). We hope you enjoy!
The Journey to Bethlehem
The story of Joseph and Mary’s hallowed journey from Nazareth to Bethlehem is an intrinsic part of christmas festivities. But what route did they take and what landscapes would they have seen?
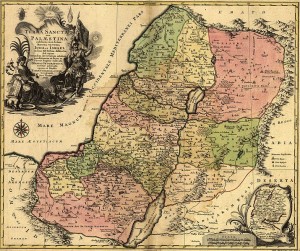
Map of the Holy Land showing the Old Kingdoms of Judea and Israel drawn in 1759. Source – Wikimedia Commons
As a distinct geographic area, the description “Holy Land” encompasses modern-day Israel, the Palestinian territories, Jordan and sometimes Syria. The geology of the Holy Land is characterised by the Judean Hills which run North to South through the centre of the region exposing Cretaceous age limestones and sandstones. The rocks reach down to the western banks of the Dead Sea and the Jordan Valley Rift valley which marks the modern border between Palestine and Jordan. The Judean Hills mark the highest area in the region (an area Joseph and Mary may have been trying to avoid!) and the topography then lowers to the Mediterranean coast to the west and the Dead Sea to the east.
Joseph and Mary’s journey to Bethlehem began in Nazareth in modern day Israel and ended in a manger in Bethlehem, which is in modern day Palestine. The route taken between the two, and indeed the time it took them is oft disputed. Given the mountainous nature of the central Holyland which is dominated by the Judean Hills and the reality of transporting a pregnant woman on a donkey, it is possible they would have avoided the mountains and travelled southeast across the Jezreel Valley, connecting with the Jordan Valley to the East, down to Jericho and then across to Bethlehem. This route would have looked something like this.

Image of the Judean Hill taken in 1917. Source – Wikimedia Commons
The area they may have wanted to avoid, the Judean Hills, is formed from monoclinic folds and relates to the Syrian Arc belt of anticlinal folding in the region that began in the Late Cretaceous. These are the same hills that include the famous Mount of Olives, and the location of the story of David and Goliath which occurred in the Ella Valley in the Judean Hills’. It is also home to Bethlehem which stands at an elevation of about 775 meters and is situated on the southern portion in the Judean Hills.
By contrast, the Jordan valley encompasses the lowest point in the world, the Dead Sea (sitting at 420 below sea level). The valley was formed in the Miocene (23.8 – 5.3 Myr) when the Arabian tectonic plate moved away from Africa. The plate boundary which extends through the valley (and houses the Dead Sea!) is called the Dead Sea Transform. This boundary separates the Arabian plate from the African plate. For more on the geology of the Dead Sea region see this earlier Four Degrees post.
Lego

Christmas tree made of Lego at St Pancras Station! Source – Wikimedia Commons
As children (or adults!) many of us will have experienced unwrapping various Lego sets on Christmas Day. Its popularity has been sustained over the last 50-60 years whilst the company has continued to grow; Lego never goes out of style! But did you know that Lego has been manufacturing its hugely successful interlocking toy bricks since 1949 and as of 2013, 560 billion Lego parts have been produced! But what does any of this have to do with geology?

Lego blocks! Source – Wikimedia Commons
Well, Lego started off as wooden blocks and toys in the workshop of inventor Ole Kirk Christiansen, before moving onto manufacuring the blocks out of cellulose acetate. But since 1963 the blocks have been made from a resilient plastic called acrylonitrile butadiene styrene (ABS). As with many plastics, the Butadiene and Styrene components of ABS are formed from a process that begins with the extraction and cracking of crude oil. Oil consists of a mixture of hundreds of different hydrocarbons containing any number of carbon atoms from 1-100. Butadiene is a petroleum hydrocarbon that is obtained from the C4 fraction of steam cracking (more on steam cracking here ) and styrene is made by the dehydrogenation of ethylbenzene, a hydrocarbon obtained in the reaction of ethylene and benzene. Lego is just another manufactured product who’s journey began in the rocks!
Wrapping Paper

Christmas wrapping paper! Source – Wikimedia Commons
The use of wrapping paper was first documented in ancient China where it was invented in 2nd century BC but it was the innovations of Rollie and Joyce Hall, the founders of Hallmark Cards that helped popularise the idea of wrapping in the 20th Century. Wrapping paper is made using specially milled wood pulp, this pulp is made from a special class of trees called softwoods. The paper is then bleached and decoration and colours are printed onto the paper using dyes and pigments.
Whilst many dyes that are used in the modern day are synthetic, originally all dye materials were sourced from natural materials. Here we focus on how to make the dyes and pigments for christmassy colours!
There are a variety of natural materials that can be used to make red dyes including lichen, henna and Madder. Madder, made from the dye plant Rubia tinctorum, has been used as a dye as far back as 1500BC it was even found in the tomb of Tutankhamun. Madder was also used to make Alizarin, the compound 1,2-dihydroxy-9,10-anthracenedione. Alizarin was a prominent red dye until synthetic Alizarin was successfully duplicated in 1869 when German chemists Carl Graebe and Carl Liebermann found a way to produce alizarin from anthracene. A later discovery that anthracene could be abstracted from coal tar further advanced the importance and affordability of alizarin as a synthetic dye. This reduced cost caused the market for madder to collapse almost overnight. While alizarin has been largely replaced by more light-resistant pigmens it is still used in some printing. (QI – it is also used in classrooms as a stain to indicate the calcium carbonate minerals, calcite and aragonite!)
Other more exotic inks and pigments used in wrapping paper such as metallic pigments are also made through mined raw materials. To produce metallic pigments, materials such as Aluminium powder (aluminium bronze) and copper-zinc alloy powder (gold bronze) are used to produce novel silver and gold inks!
Christmas Trees

Abies Nordmanniana on sale as christmas trees. Source – Wikimedia Commons
Christmas trees are an iconic part of Christmas, whether at home or in your local area its hard to go far in December without seeing one most days! In fact they are so popular now that Christmas trees are farmed specifically for this purpose. While the best selling trees in North America are Scots Pine, Douglas-fir and noble fir, in the UK, Nordmann fir is the most popular species due to its low needle drop feature.
As with all crops, Christmas trees require a specific set of nutrients to thrive and these are provided by fertile soil which is controlled by the underlying geology. Elements that are required for health growth include Nitrogen, Phosphorus, Potassium, Calcium, Magnesium, Sulphur, Boron, Copper, Manganese, Molybdenum, Iron and Zinc which are all obtained from the soil.
Where this isn’t available or in areas of intensive farming these elements are derived through the use of fertiliser which relies on mined phosphate for mass production (more on the link between fertiliser and mined phosphate reserves here). In terms of soil types, pine trees are usually better adapted to a sandy or sandy loam soil, while White Spruce trees and fir trees, prefer fine-texture loams and clay loam soils.

Abies nordmanniana trees located in the Black Sea region of Turkey. Source – Wikimedia Commons
The popular Nordmann fir used in the UK, or ‘Abies nordmanniana’ is native to the mountains to the east and west of the Black Sea in an area which covers Turkey, Georgia, Russian Caucasus and Armenia. They grow at high altitudes of 900-2200 m on mountains and require plenty of rainfall (~1000mm).
The distribution of the species around the Black Sea and its absence in other local areas of similar, suitable climate is thought to be due to the forest refugia that formed during the ice age. Refugia is the term used to describe a location of an isolated or relict species population. This can be due to climatic changes, as with Nordmann Fir, geography (and therefore geology) or human activities such as deforestation. The forest refugia that caused the limited spread of the Nordmann Fir was caused by the glacial coverage during the Ice Age in the eastern and southern black sea which cut off many areas restricting the spread of the species. Indeed the presence of these refugia is the reason many forest tree populations survived at all!
Stay tuned for Marion’s Part 2 of the Christmas Post next week…
Flo