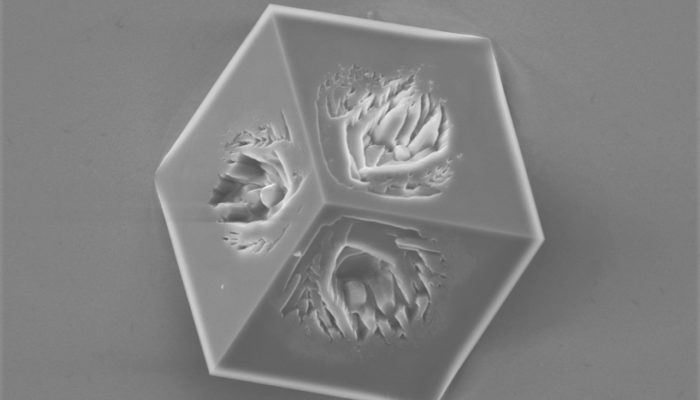
This imperfect cube of calcite was formed on the gold surface during the electrochemically assisted scaling process. Once negative electrochemical potential is applied to the gold surface, the oxygen dissolved in water undergoes reduction, yielding hydroxide anions. These anions accumulate at the gold-solution interface, forming a high pH layer. As calcite becomes less soluble with the increasing ...[Read More]
Imaggeo On Monday: Wannabe cubic calcite