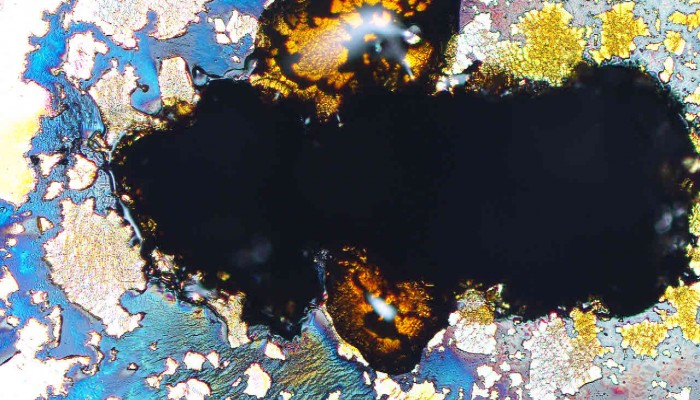
Deciphering the past history of rocks and what they might reveal about the Earth’s future is a key part of geology, and tools such as Ion Probes can be used by Earth Scientists to extract valuable information about a rock’s past. Today’s Imaggeo on Monday’s image was acquired by Sarah Glynn, a researcher at the University of the Witwatersrand, in South Africa, who was analysing a potential calcite ...[Read More]
Imaggeo on Mondays: Electron cloud gone wrong