After a short winter break, the Geochemistry, Mineralogy, Petrology and Volcanology division’s early career scientists talks (EGU campfires) are back! The first session in 2022 (and 16th overall) will be a general format event. The talks will be held on Wednesday 16th February at 4pm CET on Zoom. Our four speakers are: Barbara Bonechi (PostDoc @ Sapienza University of Rome, Italy) – High pre ...[Read More]
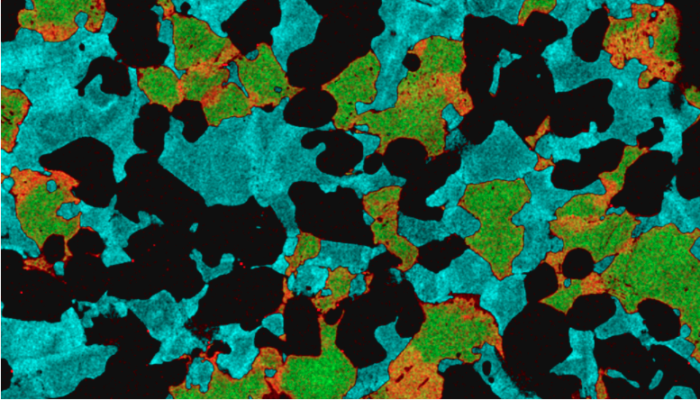
Are mantle melts heterogeneous on a centimeter scale?
The mantle makes up the majority of the volume of the Earth, but there is still a lot about it that we don’t understand. This is because we can’t observe it directly – forget ‘Journey to the center of the Earth’ – even our deepest drill holes (about 12 km deep) are merely tickling the surface of the planet (about 6400 km to the center). Most of what we know abou ...[Read More]
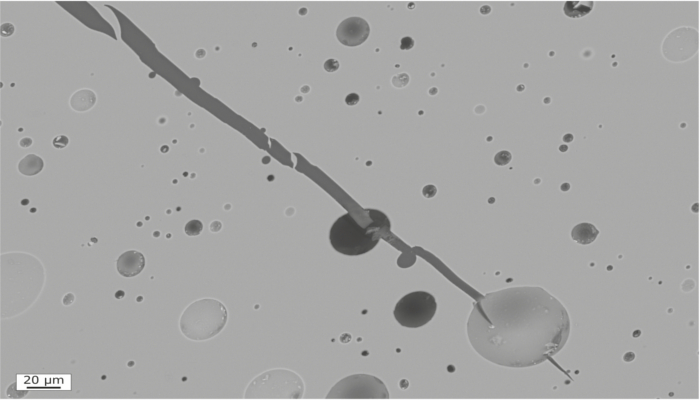
A little fracture can go a long way: How experiments illuminate our understanding of volcanic eruptions
What controls how violently a volcano erupts? Stratovolcanoes like Mount St Helens (USA), Gunung Merapi (Indonesia), or Volcán de Colima (Mexico) tend to erupt in two distinct ways: effusively and/or explosively. Effusive eruptions are eruptions where lava is extruded without any major explosions. Although effusive eruptions can be dangerous, at stratovolcanoes they tend to be restricted to volcan ...[Read More]