After what has seemed like an eternity (or more than one post-doc post, which is essentially the same thing), I’ve got a new paper published. To commemorate this auspicious occasion, I’m going to write about it.
The paper is published in Atmospheric Chemistry and Physics and is available open access here.
The study investigated how aerosol particles can alter atmospheric chemistry during the night using measurements on-board a research aircraft flying around the UK. These alterations are important as they can affect the build-up of pollution both during the night and into the following day. These changes can worsen air quality and affect regional climate. Most of the previous studies on this topic have been done in the USA, where the chemical make-up of aerosol particles is often quite different to European aerosol particles.
Specifically, the study looked at how a gaseous chemical compound called dinitrogen pentoxide, which is chemistry-speak for two nitrogen molecules and five oxygen molecules (N2O5), reacts with aerosol particles. Under the right conditions, N2O5 can interact with aerosol particles and be ‘captured’ by them via something called a heterogeneous reaction. This ‘uptake’ of N2O5 can lead to reactions with the water commonly associated with aerosol particles, form another compound called nitric acid and finally join the particle realm as nitrate aerosol.
Nitrate aerosol is a pollutant, so this process has ramifications for air quality plus nitrate aerosol is very good at bouncing sunlight back to space, which cools the surface of the Earth. Furthermore, under certain conditions, this process can enhance ozone formation in the lower atmosphere. Ozone is bad for our health if we breath it in and it is a greenhouse gas, so it warms the atmosphere.
The night-time bit of the equation is important as N2O5 is only present in the atmosphere in significant quantities during darkness; during the day it is destroyed by sunlight. There haven’t been many studies looking at this process in the real atmosphere and very few have been done on an aircraft.
A hard day’s night
Flying on the aircraft was an interesting experience as we were typically taking off around 10pm and not landing again until gone 2am (dirty stop outs that we were). Personally, I typically had another hour of work to do on the ground after a flight as I had to calibrate the instrument I was running, which meant I generally didn’t get back to the hotel until after 3am. This went on for two weeks, which meant we had to adjust our sleeping patterns accordingly. This was drastically different to any other flying campaign I have worked on, where we generally have to be on the aircraft between 4-6am to turn on the instruments. Bizarrely, I’ve never felt so rested during a project, as I typically got around 8 hours sleep every night!
So, what did we find out?
- We found that the chemical make-up of the aerosol particles influenced night-time atmospheric chemistry. The strongest controls were the amount of water associated with the aerosol, which increased the uptake of N2O5 to the particles, and the amount of ammonium nitrate in the aerosol, which decreased the uptake of N2O5.
- Compared to previous studies, the level of uptake of N2O5 was relatively efficient, which was probably a result of air being moister in our study region. The increased moister leads to more water being available to condense onto the aerosol particles, which will promote this uptake process.
- When we compared our measurements to parameterisations that have been proposed for regional and global aerosol models, the parameterisations didn’t do very well. These parameterisations are equations that have been put together based on laboratory studies of the N2O5 uptake process.
These points are summarised in the graphic below, which shows a comparison of the parameterised and measured N2O5 uptake, which is commonly shortened to γ(N2O5). The horizontal axis is for the measurements, with the vertical axis for the parameterised values. Each sub-figure is for a set of calculations using different parameterisations and assumptions and the scales are the same in all of the plots. The markers are coloured according to the ratio of water to nitrate in the aerosol and the little bars denote the variation in each data point. Brighter colours are when water strongly outweighs nitrate, which typically occurs at greater N2O5 uptake values, while darker values are when the nitrate influence is stronger and N2O5 uptake is reduced.
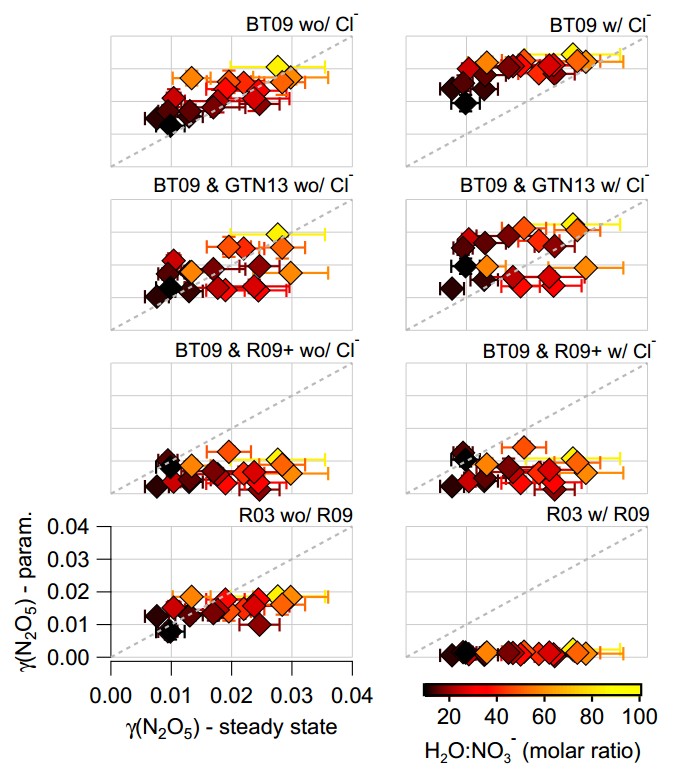
Comparisons between various parameterisations of N2O5 uptake and measured values from the aircraft. For more details, see Figure 6 in the original paper.
On the whole, the parameterised values ranged from OK to terrible when we compared them with the measurements. I should point out here that the parameterised values are calculated using the measured aerosol particle properties (size and chemical composition), alongside measurements of temperature and relative humidity. There are various assumptions required to do the calculations, although these are effectively the same as those that would be used in an actual aerosol model.
You shook me all night long
If we truly understood the N2O5 uptake process, then we would expect very reasonable agreement between the parameterisations and the measurements. The parameterisations give very different answers, so this isn’t just a case of the parameterisations being in one corner and the measurements being in another.
Scientifically, this is troubling, as we think we understand many of the underlying processes in the laboratory but these do not appear to translate to the real world. It may be that the relatively simple aerosol particles that are tested in the lab, which typically only contain one or two chemical components under closely controlled conditions, are not representative of the much more complicated particles we measure in the real world.
The parameterisation that performs the ‘best’ is the one in the top left, which is based on the water-to-nitrate ratio only. This broadly captures the variation in the measurements and the values are similar. However, this is another troubling outcome as we know that this is incorrect based on previous evidence from laboratory and field studies. We know that other aerosol chemical components, particularly organic aerosol, should be playing a role here but this parameterisation ignores it. If we do include organic aerosol, the parameterised N2O5 uptake is strongly under-predicted compared with the measurements.
This is an example of being ‘right’ for the wrong reasons. Something is missing from our understanding of how this complex system behaves. We’re not the only group to have seen this, although I would say we’ve done the most thorough look at a range of different parameterisations and assumptions.
Unfortunately, this is one of those scientific papers that provides problems rather than solutions. My hunch is that we need to study the N2O5 uptake process using more complex aerosol particles, both in the lab and field, in order to figure out some of these issues.
How much all of this matters is a good question. The upshot of the paper is that we currently don’t have a particularly good grasp of this process, which might have ramifications for regional and global aerosol model studies. We’ve actually got a paper coming out soon looking at some of these issues at the regional level, so hopefully some of the answers aren’t too far away.
—————————————————————————————————————————————————————–
I would like to thank the RONOCO (ROle of Nighttime chemistry in controlling the Oxidising Capacity of the atmOsphere) project, which as well as being a terrible acronym, was a great team of people to work with. Many thanks to all of the folks associated with the BAe-146 research aircraft used during the project. Also, the Natural Environment Research Council (NERC) funded the work.
Header image: London, England at Night published on the NASA Marshall Flickr. The photograph was taken by astronaut Chris Hadfield on February 2, 2013 from the International Space Station. We flew around London on a couple of occasions during the project, which was a pretty cool sight during the night.