There is a new commentary piece in Nature Geoscience by Gavin Schmidt and colleagues on ‘Reconciling warming trends’. The paper investigates several potential causes for the discrepancy between climate model projections and the recent ‘slowdown’ in global surface temperatures, which is nicely illustrated on Ed Hawkins’ Climate Lab Book blog.
One of the aspects the paper looks at is the influence of aerosol particles in the troposphere (the lower part of the atmosphere), which tend to exert a cooling influence on our climate. Of the aspects they looked at, aerosol particles are the most uncertain.
As I summarised in this previous post, the final report from the Intergovernmental Panel on Climate Change (IPCC) on the physical science basis concluded that aerosols was continue to dominate the uncertainty in the human influence on climate. They said that a complete understanding of past and future climate change requires a thorough assessment of aerosol-cloud-radiation interactions.
With this in mind, I want to delve into the details of how the chemical make-up of aerosol particles varies across the globe and how this is important for our climate and how this relates to the warming trends paper.
What are aerosols made of?
Aerosols are made up of a large variety of different chemical species, which vary by the time of day, seasonally, regionally and a host of other factors. There is no single type of aerosol in our atmosphere.
The figure below attempts to pull together a huge collection of studies that have been measuring the major aerosol chemical species in different regions of the globe. We can see on the map that many of the sites are in North America and Western Europe, where large coordinated surface networks have been making such measurements for decades. Measurements elsewhere are much more limited, particularly in the Southern Hemisphere.
This is one of the major difficulties in understanding aerosols; they are difficult to measure, the techniques used are typically quite labour intensive and they require access to well developed infrastructure and supporting measurements.
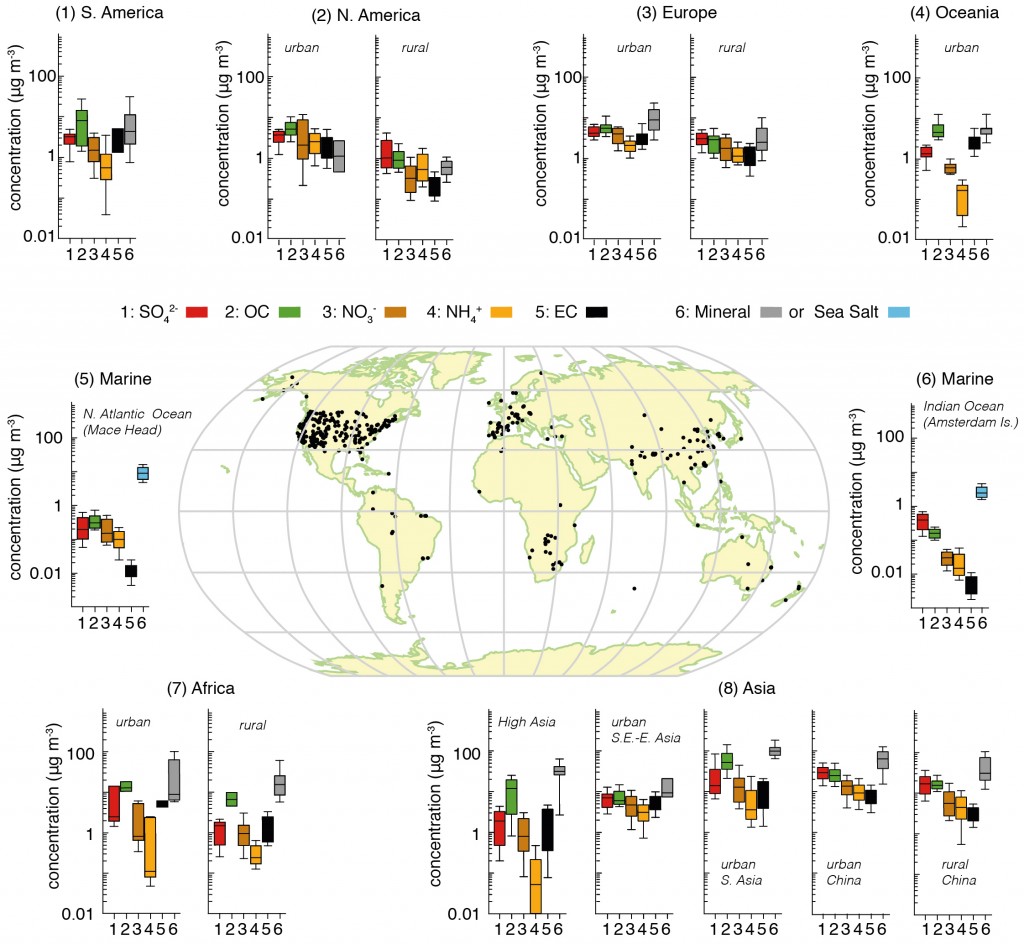
Summary of aerosol chemical species split across different regions based on surface measurements. The map shows the locations of the sites. For each area, the panels represent the median, the 25th to 75th percentiles (box), and the 10th to 90th percentiles (whiskers) for each aerosol component. The colour code for the measured components is shown in the key where SO4 refers to sulphate, OC is organic aerosol (strictly organic carbon), NO3 is nitrate, NH4 is ammonium, EC is black carbon (strictly elemental carbon), mineral refers to mineral dust e.g. from deserts and sea-salt refers to aerosols produced by ocean waves. The figure is 7.13 from the IPCC report, where the numerous references for the data are included. Click on the image for a larger view. Source: IPCC.
Bearing this in mind, we are able to draw some broad conclusions on their chemical make-up. We can see that in all of the non-marine regions, the aerosol is broadly made up of sulphate, organic carbon, nitrate, ammonium and black carbon, with the influence of mineral dust varying depending on location e.g. concentrations are much greater in locations such as Africa and Asia where large deserts exist.
Of these species, sulphate, nitrate, ammonium and black carbon are predominantly man-made in origin, while organic carbon can be a consequence of both natural and man-made emissions. We see that concentrations of organic carbon and nitrate are broadly equivalent to those of sulphate, with organic carbon being much greater in South America, Oceania, Africa and parts of Asia.
Where is the aerosol?
One of the key properties of aerosol particles in the troposphere is that they have a short lifetime, as they are removed from the atmosphere by rain or simply by crashing into things (terrible drivers those aerosol particles). This typically means that the largest concentrations occur near their sources; they are regionally very important in a climate context but their impact evens out at larger scales, although they do still exert a global influence.
Below is an illustration of this based on satellite measurements combined with a forecast model system, which shows ‘hotspots’ of aerosol particles over China, India and Southern Africa in particular. The metric used here is the aerosol optical depth, which is a measure of the amount of aerosol present in a vertical slice through the atmosphere.
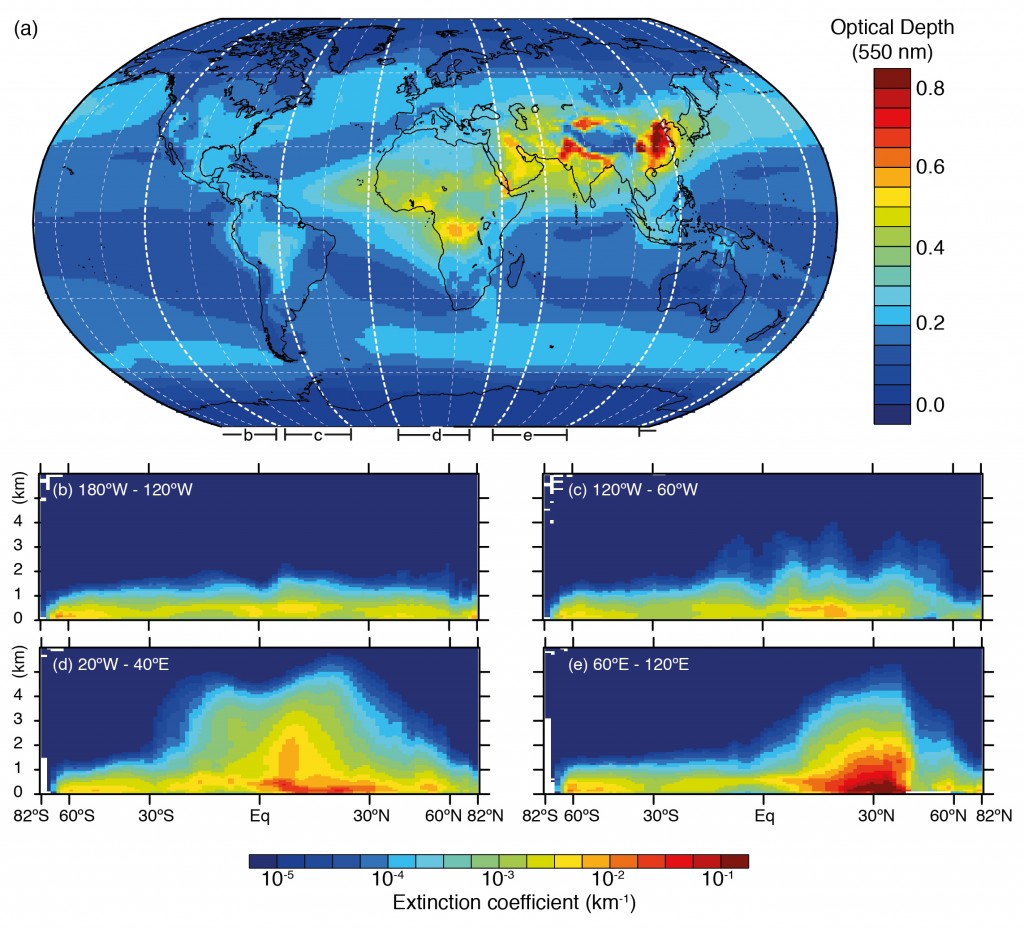
Horizontal and vertical distributions of aerosol amount based on a combination of the European Centre for Medium Range Weather Forecasts (ECMWF) Integrated Forecast System model with satellite measurements from the Moderate Resolution Imaging Spectrometer (MODIS) averaged over the period 2003–2010 and the Cloud–Aerosol Lidar with Orthogonal Polarization (CALIOP) instrument for the year 2010. The figure is 7.14 in the IPCC report and references for studies are available there. Click on the image for a larger view. Source: IPCC.
Comparing the two figures, we see that the enhanced aerosol optical depth in Asia is consistent with the large aerosol concentrations measured by the surface based networks. Of the man-made species, organic carbon is a big driver here, with nitrate, sulphate and ammonium also playing a major role. These aerosol particles are formed due to the substantial emissions from the region.
What about the pause?
Of these species, sulphate and black carbon are the major species that climate models have historically focussed on. Organic carbon is generally included, although there are large discrepancies in relation to measurements, particularly related to the secondary organic aerosol component. Nitrate has received comparatively little attention and those that have included it have shown that it is an important aerosol species both today and over the 21st Century. Drew Shindell and colleagues when comparing aerosol optical depth from satellite measurements and climate models concluded that:
A portion of the negative bias in many models is due to missing nitrate and secondary organic aerosol.
I’ve been involved in research showing how nitrate is important in North-Western Europe and how it enhances the aerosol radiative forcing in the region (many others have done so also by the way). Nitrate has long been known to be important in North America, particularly in California, where large urban and agricultural emissions coincide. Several studies have investigated its importance in East Asia, showing that it is particularly prevalent during the winter.
The ‘Reconciling warming trends’ commentary notes that nitrate was only included in two of the models that contributed to the Coupled Model Intercomparison Project (CMIP5) and that including this across all models brings them closer to the observed surface temperature trend. They also note that underestimating the secondary organic aerosol contribution would have a similar effect but don’t put a value on this.
While this certainly isn’t the last word on the importance of these species for climate change and research on the ‘pause’ will undoubtedly continue, further recognition of their contribution to atmospheric aerosol is a step in the right direction. There are many unanswered questions in this realm, particularly relating to the large differences across climate models in terms of what contribution each species makes to regional and global aerosol.
Bringing the aerosol measurement and modelling communities together more to address such issues is likely the way forward. This is a big focus in many aerosol related research projects these days…we’re trying, honest.