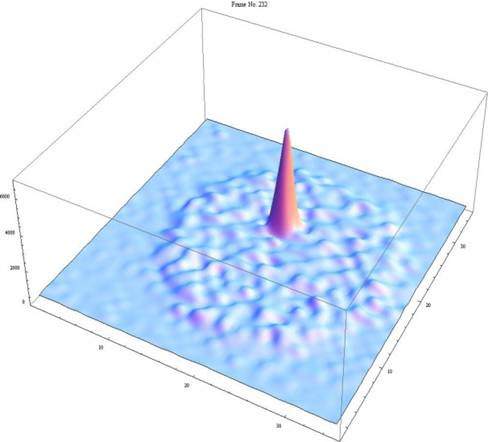
I recorded the video above on a recent field camp near Deep River, Ontario. This video shows a great example of a flowing artesian spring which is bubbling up at the headwaters of a creek. The water is freezing, crystal clear and totally delicious! The classic textbook on groundwater, Freeze and Cherry, puts the attraction of groundwater springs nicely when they say “Flowing wells (along with springs and geysers) symbolize the presence and mystery of subsurface water, and as such they have always evoked considerable public interest.”
There are two types of artesian springs. Those that are controlled geologically, which are commonly taught as the only variety of artesian system, and topographically, which are often overlooked.
Geologically controlled artesian springs/wells result from a specific combination of hydrogeologic conditions. Specifically, the aquifer must be under pressure, which is usually caused by a steep elevation gradient in combination with relatively impermeable confining layers such as clay. This is called a confined aquifer. Recharge to this aquifer occurs on top of a hill, where the aquifer outcrops. This water then infiltrates through the permeable sediments to the water table and into the confined aquifer. However, this does not explain why a spring or a well drilled into and artesian aquifer often bubbles up with water, like the video above.

A conceptual model of a confined artesian aquifer in which the recharge area is exposed at higher elevation and the aquifer sediments are bounded by two aquitards. Source
The reason for this is somewhat abstract and has to do with water pressure. In an unconfined aquifer the water table and the potentiometric surface, which is the abstract line dictated by the water level in the well, are generally synonymous and are defined by the point at which the water pressure is equal to atmospheric pressure. However, in confined aquifers where artesian conditions exist this becomes more complicated. The reason for this is that within the confined aquifer the water pressure is often greater than atmospheric. Imagine diving down in a lake and feeling the pressure of the water above you. Therefore, when this aquifer is drilled or a pathway to conduct water to the surface exists the water will want to flow upward towards that point where the water pressure and the atmospheric pressure are equal. This point can be above the ground surface and this leads to flowing artesian conditions. The figure below illustrates this concept nicely.
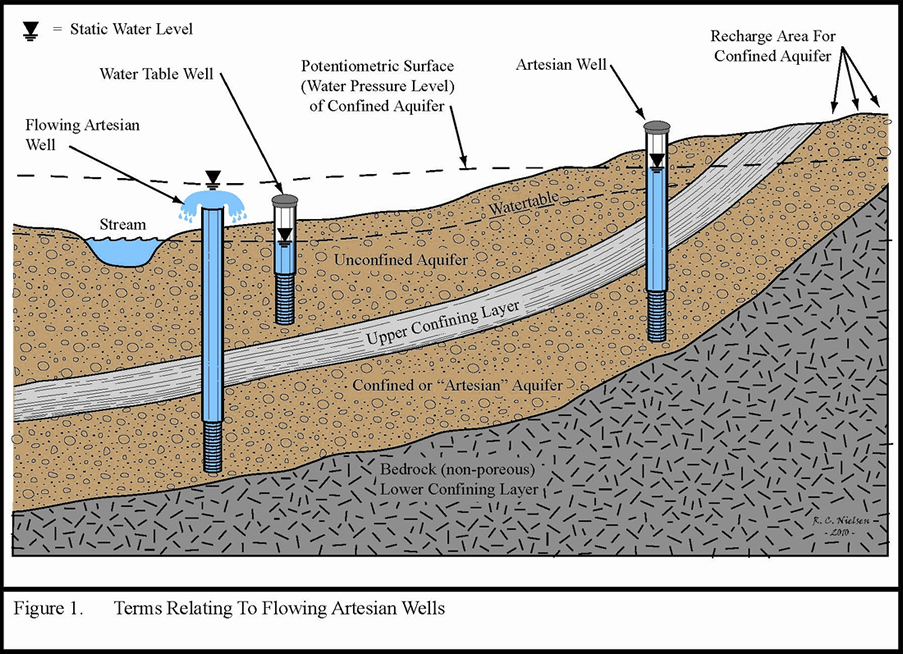
Source: Minnesota Department of Health,
In this figure the water level in the well on the right, which is connected to the confined aquifer, is distinct from the water table in the unconfined aquifer. The water is not flowing because the potentiometric surface is not higher than the ground level. In the other artesian well, which is flowing, the water flows up to the potentiometric surface, well above the ground surface. This is because that surface represents the point where the water pressure, which is the pressure of the water within the confined aquifer, and the atmospheric pressure are equal.
The other type of artesian spring are topographically controlled and often occur in valleys. The reason for this is that as water recharges at the top of hills this can locally raise the potentiometric surface if there is a steep valley nearby. Therefore, at the base of the valley the potentiometric surface can be higher than the ground surface causing water to discharge.
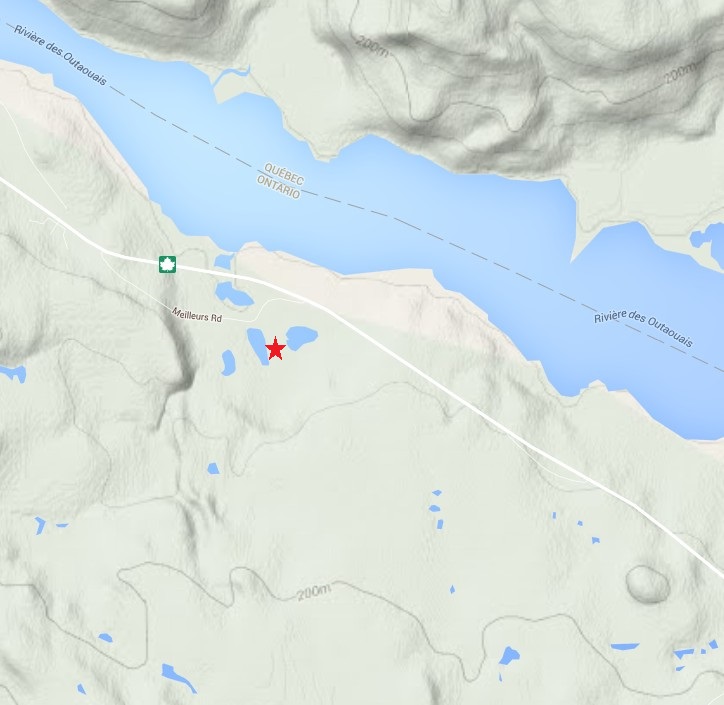
So which type is the one in the video? Let’s start by checking the topo map of the region. The spring is located at the red star, which based on the terrain map is actually pretty flat, certainly much flatter than the opposite bank of the Ottawa river.
Based on this map it doesn’t look like the spring is topographically controlled. There may be some local elevation that does not show up at the map scale, although I don’t recall there being that much. One thing to keep in mind about this location is that there is a lot of bedrock exposed. It is possible that some of this bedrock aquifer is over-pressured and water flowing through fractures in the bedrock is discharging as a flowing artesian spring. In my mind, after about 10 minutes of looking around, this is the most likely scenario. It may also be completely wrong, but without a more detailed look around it is difficult to say.
Artesian springs and springs in general really represent the importance of protecting our groundwater resources. It is critical that places such as this artesian spring be protected from contamination and development as they are very fragile and represent important sources of clean, safe water as well as habitat to a large diversity of local flora and fauna. If you know of any artesian springs in your area please comment below and let me know if they are protected or if they have been compromised by contamination or development.
Matt
p.s. I’ve teamed up with Science Borealis, Dr. Paige Jarreau from Louisiana State University and 20 other Canadian science bloggers, to conduct a broad survey of Canadian science blog readers. Together we are trying to find out who reads science blogs in Canada, where they come from, whether Canadian-specific content is important to them and where they go for trustworthy, accurate science news and information. Your feedback will also help me learn more about my own blog readers.
It only take 5 minutes to complete the survey. Begin here: http://bit.ly/ScienceBorealisSurvey
If you complete the survey you will be entered to win one of eleven prizes! A $50 Chapters Gift Card, a $20 surprise gift card, 3 Science Borealis T-shirts and 6 Surprise Gifts! PLUS everyone who completes the survey will receive a free hi-resolution science photograph from Paige’s Photography!