It is once again time to write about geology and classics and the incredibly important impact the geosciences had on the ancients and their way of life. My previous post on this topic can be found at my old blog location as the post: The Odyssey and Geology. I’ll begin by relating a story:

Themistocles (Wikimedia Commons)
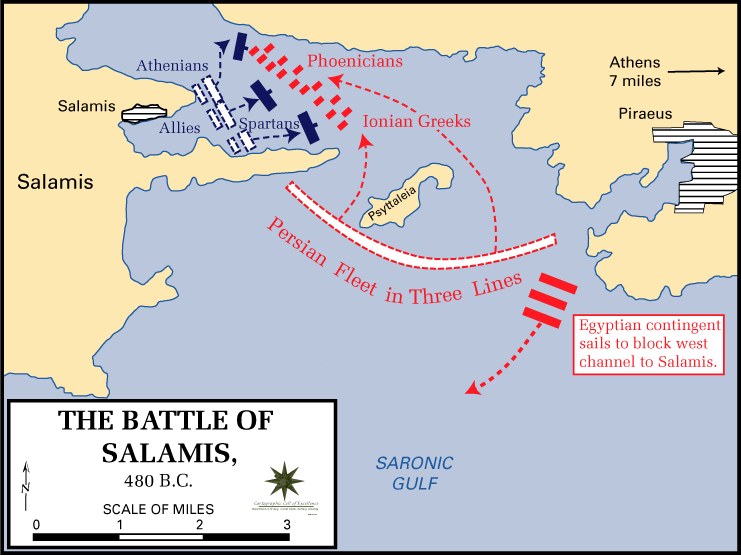
The two fleets, the Persians the the Greeks, which was composed of the navies of all the city states, but mainly Athens, met in the narrow Strait of Salamis for a final and deciding battle. The Persian king, Xerxes, was so certain of victory that he set up a throne in nearby Athens to watch the battle. The true architect of the battle though was not Xerxes, but the Athenian Themistocles, a politician and general. Indeed, it had been Themistocles who had convinced the Athenians to build a navy of over 200 additional ships in the years prior to the war and it was he who stationed the Greek navy in the Strait of Salamis, which was advantageous for the smaller Greek navy. The battle proceeded according to Themistocles’ plan and the Greeks were able to out-maneuver the Persians in the narrow strait and succeeded in decimating the Persian navy. This was a great blow to Xerxes who fled back to Persia with much of his army. The next year the Greeks were able to defeat the remainder of the Persian army driving them out of Greece and winning the war. Salamis was the turning point of the war and saved Greece from certain doom.In 480 BC the ancient Greeks were faced with their biggest threat in history: the invasion of the Persian king, Xerxes and his armies. The Greeks, despite being horribly outnumbered had fought bravely but been defeated in the Battle of Thermopylae. The Persians then advanced through Greece nearly unchecked and conquered Athens. However, the Persians knew that to fully conquer Greece they would have to do so at sea as well as on land. Things were looking pretty grim for the future of ancient Greece at this point.
 |
| Source: Wikimedia Commons |
The hero of Salamis, Themistocles was a very persuasive politician as well as tactician. In fact, it was he who convinced the Athenians to build the 200 triremes (ships) that made of up the bulk of the Greek navy and can be credited with saving Greece. The credit cannot be only given to Themistocles though. In fact, the idea came from the Oracle of Delphi. The Greeks were very worried about the impending Persian invasion and decided to consult the oracle for advice on how to win the war. The oracle cryptically answered along the lines of the “wooden wall will save you”. Clearly, this answer could not refer to the building of an actual wooden wall since that would be stupid and obviously could burn down. Themistocles interpreted the oracles advice to mean build a navy. Unfortunately, the ancient Greeks had the same problem with national defence requisitions as governments do today and money for such a venture was not easily at hand. However, geology was going to come to the Greeks rescue in the form of a major silver discovery at Laurium, a small mining community just south of Athens, providing the Athenians with enough money to afford a new navy. Without the discovery at Laurium it is possible the navy would never have been built and ancient Greece would have fallen into Xerxes hands. Ergo, geology saved ancient Greece from total domination by the Persian empire.
So now what about Laurium?
 |
| Miners in Laurium. They were all slaves. (Source: Wikimedia Commons) |
 |
| Google Maps |
The Laurium silver mines are located just south of Athens and are world renowned for the excellent mineral samples the area still produces in addition to its storied past. The mineralization is of lead, zinc and silver and is associated with the emplacement of an igneous body within metamorphosed sediments. The ore occurs mainly within marble especially at the contact with other metamorphic or igneous rocks (www.mindat.org). The list of minerals found in the Laurium mining district is a mile long and it is actually the type locality for about a dozen minerals as well, making it a world class mineralogical site. The mineral Laurionite is named in honour of the location. In addition to the minerals occurring in the mines there are a host of others that occur in the ancient slag piles left by the Greeks. The slag has reacted with the sea water used in processing the ore in ancient times to produce a suite of new and unusual minerals there as well.
 |
| Agardite, Laurium, Greece (Source: www.mineral-forum.net – Used with permission) |
 |
| Diaboleite, Laurium, Greece (Source: www.mineral-forum.net – Used with permission) |
 |
| Conichalite, Laurium, Greece (Source: www.mineral-forum.net – Used with permission) |
 |
| Nealite, Laurium, Greece (Source: www.mineral-forum.net – Used with permission) |
 |
| Annabergite. Larrium, Greece. (Source: www.mineral-forum.net – Used with permission) |
The ancient ore processing techniques of the Greeks were obviously primitive relative to today’s, however, they were still able to extract both lead and silver from the ore. The process essentially involved numerous washes with water in a sloped basin. The heaviest material was collected and smelted, and the resulting slag was carted away and dumped. Since then the slag has had a few thousand years to oxidize and this process has resulted in the growth of all sorts of interesting minerals like the ones pictured above. Laurium is still geologically relevant today from the perspective of the mineralogist or mineral collector as the area still produces some world class specimens.
It has always amazed me how deeply geology is integrated with our lives today, what with our dependence on natural resources for nearly everything. However, it would appear that this is not a new phenomenon and that geology always has been and will always be a crucial part of our lives.
Thanks for reading!
Matt











