Flo Bullough discusses the meaning of good and bad water quality, what’s in our tap water and what policies control the content of drinking water.
Pressure on water supply and quality has been high on the public and media agenda over the last 18 months. The widely publicised drought in early 2012, recent reports that we are due to run out of clean water in this generation and the controversy around potential contamination from fracking and the water industry itself have got a lot of people talking about good and bad water quality.
Water is the most important, interesting and unique compound on the planet. In addition to its necessity for all life, it also plays a fundamental role in industry and agriculture. For this reason the provision of reliable supplies of water of an adequate quality is imperative for health and for the economy. Geoscientists and hydrogeologists are involved at many stages in the water provision process, including characterising the geology and geochemistry of groundwater and surface water systems, identifying contaminants and the development of materials for the remediation of polluted drinking water.
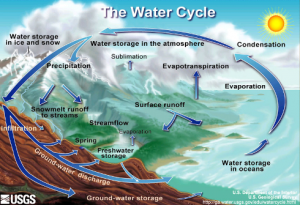
Water carries the signature of the many parts of the water cycle it has passed through and so is never as simple as H2O. Source – USGS, Wikimedia Commons
What’s in our water?
For a water scientist, the purity of water is considered differently depending on its intended use. For the purpose of this post, I’ll be looking at what’s required to make drinking water ‘fit for use’ which applies to all water intended for human use. 100% pure water is the compound H2O, but in nature, however, this doesn’t exist as water acts as the universal solvent and is often a much more complex and heterogeneous mixture of dissolved salts, inorganic and organic compounds and bacteria. This is due in part to the extremely variable nature of the geology and soil that water interacts with. Water is as unique and variable as the geological strata through which it passes creating a fingerprint of the local geology.

The dissolved minerals in water such as magnesium and calcium bicarbonate is what clogs up our kettles! Source – Julo, Wikimedia Commons.
It is the impurities it collects along the way that make the difference between hard and soft water; hard water contains more calcium and magnesium bicarbonate (leading to that nasty build up in your kettle!) but has no negative impact on health. Many of these impurities are either completely harmless or exist at concentrations below those posing a risk to health.
Some of these components create better tasting and higher quality water such as a balanced amount of magnesium, potassium, calcium and silica – chlorine, on the other hand, often ruins the taste of water. In addition to the compounds and chemicals that enter the water system in the environment, many water companies also add certain chemicals to drinking water. At the disinfection step, chlorine is often added to remove harmful bacteria, and some of this chlorine remains in the water until the point of consumption to ensure the water remains fresh as it makes its way through the pipe network. An issue of long-standing controversy is the addition of fluoride to drinking water. Fluoride is typically added to reduce tooth decay but its wider impacts have been debated extensively. Fluoride is a complex issue in that it can have beneficial health impacts at low concentrations (0.5 – 1 parts per million (ppm)) but acts as a contaminant at slightly higher doses. The threshold set by the World Health Organisation (WHO) guidelines is currently 1.5 ppm. The intense debate around this has led many countries to stop artificial fluoridation of their drinking water.
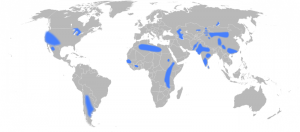
Areas with groundwater fluoride concentrations above 1.5ppm. Source – Eubulides, Wikimedia Commons.
It is largely not practiced in Europe with the exception of Ireland, Spain, Switzerland and the UK, where approximately 5.8 million people receive artificially fluoridated water. This variability in the UK is accounted for by the differing regional policies on fluoridation.
How do we define contamination and where do contaminants come from?

Some water contamination is very colourful and visible such as Acid Mine Drainage seen here in Rio Tinto, Spain. Source – Carol Stoker, NASA, Wikimedia Commons.
Contamination can occur in many forms. It may or may not be visible to the naked eye and it can occur at very low concentrations (in the parts per billion range) or at much higher concentrations. This has implications for the wider public and policy-makers in communicating and understanding the issues around water contamination and decontaminating drinking water since the presence of contamination is often far from obvious. Contamination in drinking water is assigned by threshold values: since at least trace amounts of most elements are found in drinking water due to reasons discussed above. It is only when the concentrations of these chemicals exceed an assigned threshold that there is cause for concern. Contamination is normally defined by comparing concentrations in freshwater to a set of pre-determined evidence-based threshold limits that protect humans, flora and fauna from harmful levels of certain chemicals. These contaminants can come from a wide range of sources: acute spills associated with point sources such as industry, acid mine drainage and landfill – often coined anthropogenic sources – but also natural sources, where contamination is geogenic is origin, i.e. naturally present at elevated of harmful concentrations. Geogenic sourced contaminants can be unleashed through the drilling of wells for groundwater as seen in the arsenic crisis in Bangladesh.
What are the current policies covering water quality and its improvement?
The restriction of pharmaceuticals in drinking water could incur high financial and energy costs. Source – LadyofProcrastination, Wikimedia Commons.
Substances which pose a risk to the aquatic environment are regulated in the Water Framework Driective at EU level and member states are requried to control these substances and prevent concentrations exceeding the threshold limit. There are currently over 50 standards to which drinking water is compared and these are listed by the Drinking Water Inspectorate (DWI) in the UK. The job of the DWI is to ensure that private water companies are producing water to the standards outlined in law. These health-based standards derive from those outlined by the EU in 1998 (with the exception of a few national limits), themselves based on strict guidelines outlined by the WHO. A recent inquiry carried out by the UK Government Science and Technology Committee investigated water quality threshold limits and the potential addition of new chemicals to the list of controlled substances. The main concerns were the inclusion of pharmaceutically-derived products to these legal thresholds, the extent of damage they cause, and the financial and energy costs of treating wastewater to remove them.
Problems with threshold limits
Clearly, the protection and decontamination of drinking water is of high importance in the context of a growing population and environmental change. However, adherence to current limits – which can be prohibitively low – and extension of the controlled substances list has complicated implications. Firstly, threshold limits can be extremely low, sometimes sitting at or below the levels which can be measured with current instrumentation. This can make the monitoring of contaminants both difficult and expensive, particularly in developing coutnries where sophisticated analysis instruments may not always be available. Additionally, many people argue that threshold limits are too low due to their basis on risk-derived limits and the lack of clear or ample toxicological or epidemiological studies to aid in deriving the standard value.
As issues of water and energy provision continue to converge in a difficult economic climate, knock-on impacts to intensive water remediation will only become a more important consideration for decision makers. The challenge now is to effectively communicate and manage the intensifying water issues associated with water security and quality in the light of economic factors, feasibility and the rising cost of energy.

