Hi readers of “Green tea and Velociraptors”, my name is Sabine Lengger, I am a scientist, and I am an avid reader of Jon’s blog too. I started out my scientific career as a chemist / biochemist, and became more and more fascinated by the fields of environmental chemistry and molecular palaeontology. Since Jon spends all day [apparently] writing his thesis these days and asked for a guest writer, I thought I could add the occasional thought on fossils from my perspective. Below there’s a little introduction to palaeontology from the organic chemist’s point of view, and the stuff that we get excited about … molecules!
Most people are acquainted with macroscopic fossils: dinosaur bones and shells and imprints of leaves for example. The detailed descriptions of their features have revealed much about their evolutionary history, just like classical botany and the analysis of physical features of plants or animals has led to a classification system. However, there are also fossil remains of much smaller size, and only visible under a microscope, such as the minute shells and skeletons of tiny algae or plant pollen. If we then zoom in even more and use the amazing tools of analytical chemistry, we can also find molecular components and the very chemical make-up of ancient life. In the right conditions, we can find preserved molecules such as lipids, which is what we call “fatty” molecules (they make up cell membranes, are used for energy storage or as biochemical “signals”). We can even find carbohydrates and DNA – a bit like the DNA in the amber-trapped mosquito from “Jurassic Park”1. However, we are not whacky billionaires trying to resurrect ancient life: we are chemical detectives using these molecules to find out more about the past.
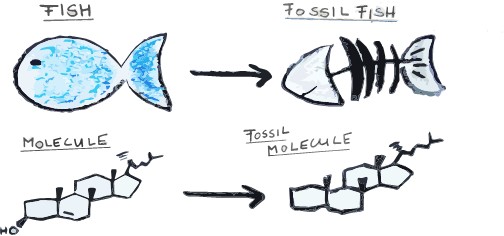
But, just like large fossils are only the decayed skeleton of a fish or a dinosaur, very often just a ‘skeleton’ of these molecules is found. So, molecular palaeontologists not only need to identify and quantify million year old molecules entombed in rocks, they also need to look at modern samples and figure out what the original molecule looked like and what organism it came from. The key component of all this is that these molecular skeletons are really specific for certain organisms, and some relate to specific environments.
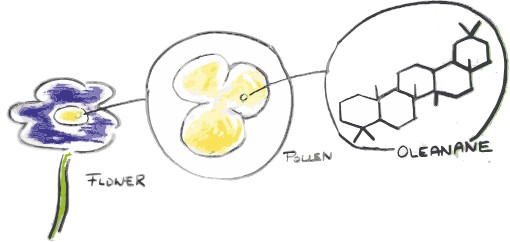
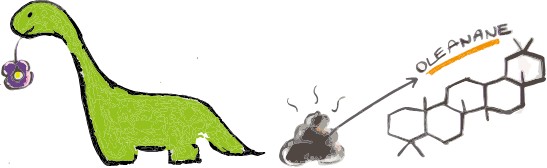
The “star” fossil molecule which can be used that way is isorenieratane. This comes from a pigment made only by certain bacteria that live in the sunlit part of the ocean, but only under anoxic conditions and under the presence of hydrogen sulphide – a very smelly ocean – and can thus tell us a lot about what the environment looked like2,3. Other famous molecules include fossilised pigments that allow us to find out about the colour of dinosaurs and their feathers4, or oleanane, which, when found in fossilized dinosaur poo (coprolites), could show that they were eating flowering plants5,6.
We’re beginning to open a new chemical dimension into the history of life on Earth thanks to the combination of chemistry and palaeontology, and I hope to bring you stories that offer a new window into ancient ecosystems.
I hope you’ll like the occasional change of perspective while Jon’s typing away!
Dr. Sabine Lengger is an organic geochemist who conducted her PhD research at the Royal Netherlands Institute for Sea Research. She has worked on archaeal lipids in sediments, contaminants in waters produced by the oil industry, on sponges and Devonian rocks, and is currently investigating lipids which can tell us more about the current and past methane cycle at the University of Bristol and lecturing Organic Chemistry at the University of Plymouth. She is into analytical chemistry, biochemical pathways, statistics, stable isotopes and likes to read about data visualization. Sabine is on Twitter (@doc_sabine), Researchgate and Google Scholar.
- D. E. Greenwalt, Y. S. Goreva, S. M. Siljestrom, T. Rose and R. E. Harbach, Proc. Natl. Acad. Sci., 2013, 110, 18496–18500.
- M. P. Koopmans, J. Köster, H. M. E. Van Kaam-Peters, F. Kenig, S. Schouten, W. A. Hartgers, J. W. de Leeuw and J. S. Sinninghe Damsté, Geochim. Cosmochim. Acta, 1996, 60, 4467–4496.
- K. Grice, P. Schaeffer, L. Schwark and J. R. Maxwell, Org. Geochem., 1997, 26, 677–690.
- J. Lindgren, A. Moyer, M. H. Schweitzer, P. Sjövall, P. Uvdal, D. E. Nilsson, J. Heimdal, A. Engdahl, J. A. Gren, B. P. Schultz and B. P. Kear, Proc. R. Soc. Lond. B Biol. Sci., 2015, 282.
- K. Chin and S. C. Brassell, in Organic Geochemistry Poster Sessions from the 16th International Meeting on Organic Geochemistry, ed. K. Øygard, Stavanger, pp. 444–447.
- A. P. Hunt, J. Milàn, S. G. Lucas and J. A. Spielmann, Eds., Vertebrate Coprolites, Albuquerque, NM, 2012.

Jonathan Priest
Thank you Sabine for your beautiful little blog. This feels like the starter, I’m now hungry for the main, and the dessert if there’s any going. Jonathan