Birds are living, breathing, tweeting dinosaurs. That is scientific knowledge backed up by overwhelming evidence, but the evidence basis for it grows strong all the time. We know that they are related from a host of morphological evidence from the last 150 million years or so. Our understanding of the origins of feathers and flight are developing too – each new finding is a piece that slots into a puzzle, where we already have a pretty good idea of what the picture we’re trying to recreate is. The evidence is mounting too with each new discovery – findings from China are rewriting the way we think about the evolution of feathers and flight, and the evolution of early birds from their dinosaurian ancestors.
One other line of evidence that palaeontologists have been drawing on, is at the embryological stage, through developmental biology (otherwise known as ontogeny). Often, this has been in the context of looking at the early embryonic development of extant birds, and comparing the features (such as feather development) to those we see in the adult forms of extinct early birds and dinosaurs. But we also have dinosaur embryos, and they provide a wealth of information too.

Embryonic skeleton of the sauropod Massospondylus carinatus. Source.
In extant birds, the skulls and jawbones contain a type of tissue known as secondary cartilage. The difference between primary and secondary cartilage is that the former arises before bone formation, and the latter arises on pre-existing bone structures. In extant birds, it forms at articulation points to smooth movement, and begins to form at the embryonic phase of growth. This is unique among extant lizards, birds, amphibians, snakes, and crocs, which all use a collagen fibres at articulation points; secondary cartilage in fish and mammals is considered to have arisen independently to birds.
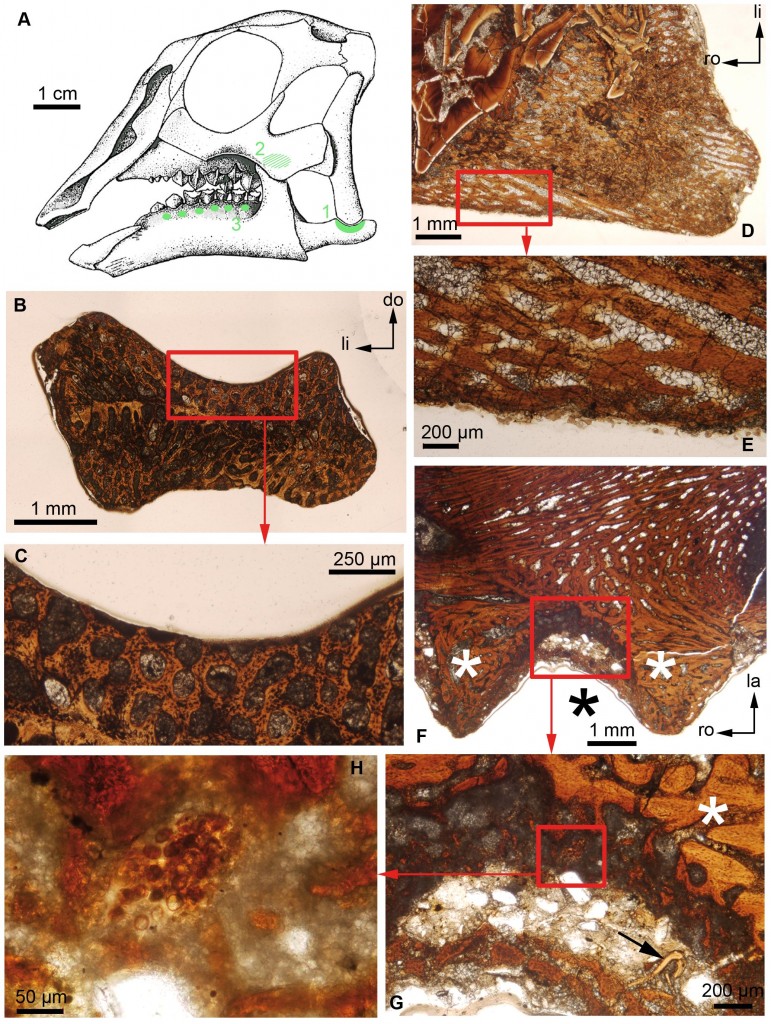
A) Reconstruction of the embryonic skull of Hypacrosaurus stebingeri, (B) Transverse section of the surangular of an indeterminate hadrosaurid, (C) Close-up of the red box in (B), (D) Section of the maxilla (cheekbone) of Hypacrosaurus stebingeri, (E) Close-up of the red box in (D), (F) Section of the dentary of Hypacrosaurus stebingeri, (G) Close-up of the red box in (F). The arrow indicates a remnant of dentine. (H) Close-up of the red box in (G). (F) and (G) show alveolar bone (white asterisks), (G) and (H) show a secondary cartilage islet. Source. (click for larger)
Secondary cartilage is known in extinct dinosaurs, specifically the adult version of Hypacrosaurus stebingeri, a hadrosaur from the Late Cretaceous of North America and renowned crested veggie-chomper. This cartilage has been established as the same sort that birds possess, and is further evidence of the link between all types of dinosaur and modern birds. But the authors of this study weren’t content with this find – they wanted to establish the connection between the two based on a developmental context, using dinosaur embryos (yeah, they exist!). To do this, they chopped open (gasp) cranial elements of three different species of dinosaur (Hypacrosaurus stebingeri, Maiasauria peeblesorum, and a specimen with unknown identity) all from specimens collected in Montana. This process is called chondrogenic osteohistology (yep), and gives a cross-sectional perspective through bones in a developmental growth context, and is very useful in looking at microstructure (which reveals a lot about age, biomechanics, and dinosaur development).
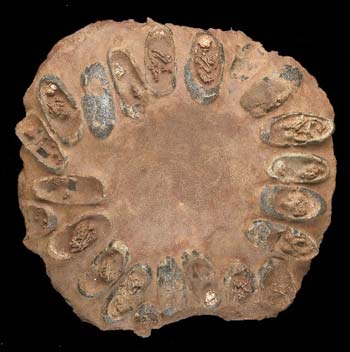
A whole nest of dinosaur eggs, with embryos still preserved within the shells. Source.
The trio found a single location with secondary cartilage in the Hypacrosaurus specimens, identified by an area of large, round cells different from the surrounding bone, and surrounded by a thick layer of dentine. It doesn’t look like they found any in the other specimens, although the study is a little unclear about this. What it does show is that, combined with the other study, secondary cartilage is present through all ontogenetic stages of growth in this species, and in birds, which means that its presence can be extrapolated back to before the origin of the branching split in dinosaurs (into saurischians (leading to birds) and ornithischians (including the specimens analysed here)). Alternatively, it implies that the origin of secondary cartilage arose independently more than once in dinosaurs, and is still present today in modern dinosaurs.

I can haz dinosaur embryo? Source.
The timing of the origin of this tissue in the Hypacrosaurus embryo is a little more difficult to calculate. In modern chick embryos, secondary cartilage arises on about the 11th day of a 21 day incubation (these things grow exceptionally fast). The size of Hypacrosaurus eggs, on the other hand, had a much longer incubation time, based on their size and the time it would take to calcify the egg. The team estimate an incubation time of 74 days, based on the estimated egg volume. Combined with the size of the embryonic bones analysed, they were probably two-thirds to three-quarters of their way through the embryonic phase, implying that there was enough time left compared to modern birds to develop additional secondary cartilage localities. What this does suggest, is that the rate of development between ornithischian dinosaurs is different to that in extant birds.
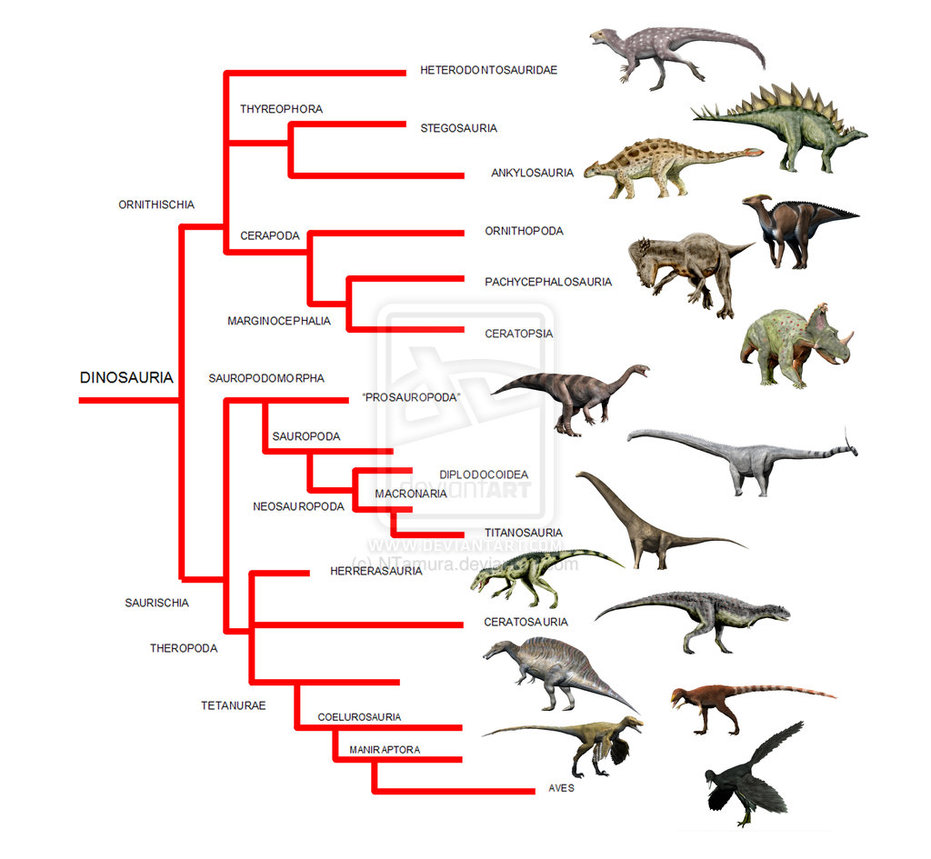
Current understanding of how the different groups of dinosaurs are related. With secondary cartilage known now in both birds (Aves) and hadrosaurs, we can say that it ether originated at the base of the tree or independently in both lineages. Source. (click for larger)
There are two reasons, according to Mr. Science, why this could be. One of the reasons is that secondary cartilage forms due to differences in skeletal motility (ability to move independently and spontaneously) at the embryonic stage, with a higher threshold of formation in ornithischian dinosaurs. Alternatively, the formation differences could be due to mechanical variations in the skull relating to the presence of the avian beak, which changes the stress regimes in the rest of the skull and kinetic capabilities of the skull joints. This in turn could relate to the function of the beak during the lifetime of an organism – i.e., birds which experience higher stress regimes (due to feeding styles, for example), would have greater secondary cartilage formation.
The authors are sensible, however, and note that this is only a preliminary study on a limited sample size with limited taxonomic scope. It would be cool to see in future how pervasive secondary cartilage is throughout other dinosaurs, to really hammer home the developmental embryological evidence for the evolutionary link between dinosaurs and birds.
Reference:
Bailleul et al. (2013) Secondary cartilage revealed in a non-avian dinosaur embryo, PLoS One, 8(2), e56937 (open access, so you can read for free!)

Gary Kaiser
Just some thoughts on the effect of egg shape on dino embryos. Not only do the elongated eggs of theropods (etc.) provide room for better development of the long leg bones of precocial young but their shape provides a greater surface-to-volume ratio so that more calcium can be extracted from the inside of the shell. More calcium is also useful if you are growing a long tail at the same time.