I don’t think it is any secret that the world is facing an imminent energy crisis. We are trying to generate more power than ever before, but at the same time, we now realize we have to do it in a sustainable way that does not harm the environment or exacerbate any existing issues such as climate change. The problem is these two goals are often mutually exclusive. Most of our power generation methods have environmental implications or are simply at the early technology stage and cannot be relied upon the produce the juice that we all need. Thus, we’re stuck between a rock and a hard place when it comes to generating power. Therefore, the question becomes: we need more power, but at what cost? What are we willing to compromise to generate the energy? The answer is generally something in the environment protection aspect. We now accept that in generating the power we need we will have to affect the environment either to obtain the natural resources required or use huge tracts of land. The trick is how do we best manage the risks and impact of power generation?

The Bruce Nuclear Generating Station in Ontario, Canada. The largest nuclear plant in the world. (Wikipedia Commons – User: Cszmurlo)
This post isn’t about energy policy though. Instead, I would like to write about how we manage the risks of nuclear power, specifically the waste? One of the big knocks against nuclear energy is what do we do with the potentially dangerous and long lived waste that it generates? Over the years a huge range of ideas have been proposed, some silly, and some practical. In this post I intend to explore some of these proposals and see which ones make the most sense given that this is a problem the world is facing now and is going to face for the foreseeable future, especially, if nuclear energy generation expands. One important point is that most radioactive waste is classified as low/intermediate level. This is essentially everything that is not heat generating fuel. The high level waste is the left over fuel. For all intents and purposes the disposal options aren’t different it’s just that high level waste seems scarier and has the potential to cause more environmental or human health damage if it escapes. Either way the waste, no matter what variety, has got to stay isolated essentially forever.
What are the key features that every storage solution must have?
1. Must be immobile/isolated from the environment and people for at least 100,000 to 1,000,000 years.
The reason for this requirement is pretty obvious. Nuclear waste is dangerous and therefore, it must be isolated from the environment for long periods of time. The reason it must be completely isolated for so long is because the half lives of the isotopes within the waste vary greatly. Thus even though the most highly radioactive isotopes decay to almost nothing within the first 1,000 years there are others, like iodine-129, which have long half lives and remain radioactive for millions of years.
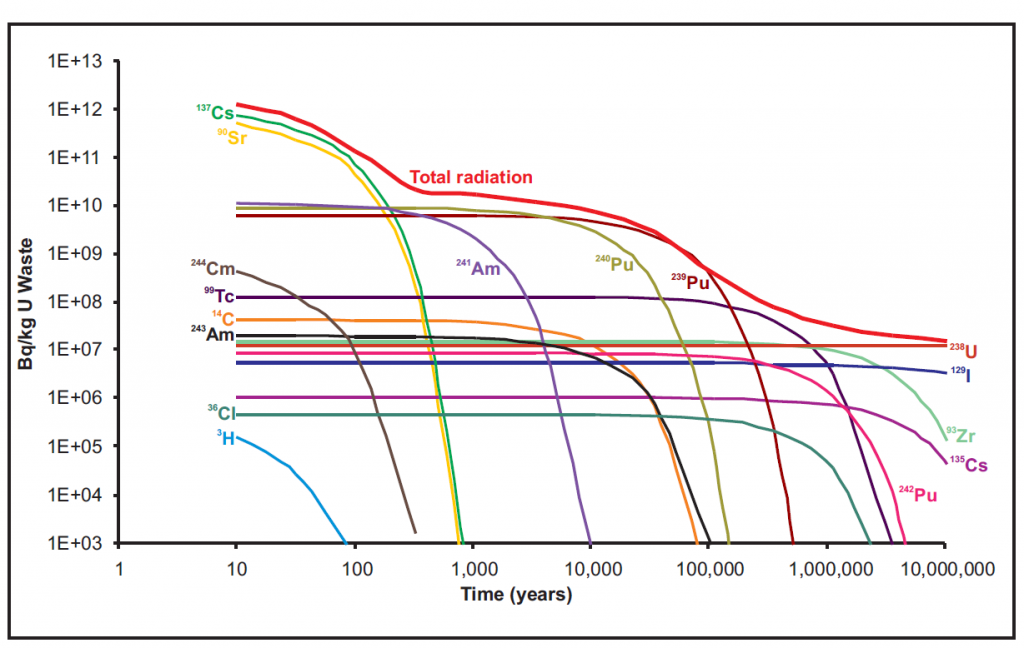
This shows the relative activity of each major isotope in high level radioactive waste and how it decays overs time. The top red line shows the total radiation over time. This way you can easily see which isotopes are contributing the most to the total radioactivity of the waste over long time scales.
2. Must be potentially recoverable.
The reason that the waste must be potentially recoverable is two-fold. Should there be a problem with the repository it must be possible to remove the waste and either fix the problem or relocate it. The second reason is that as nuclear technologies advance it may become feasible to re-use the waste for energy generation or some other purpose. Therefore, the waste must remain isolated yet accessible during the lifetime of the repository.
3. Must be able to withstand every possible circumstance for incursion.
This one is clearly a key characteristic of a repository. This waste can be used for potentially nefarious reasons and thus it cannot be simple to access. Furthermore, given that the lifetime of the repository is far greater than human civilization has already existed it must also be able to withstand unintentional incursions by future generations. For example, future generations may not use existing languages. Therefore, it must be communicated unmistakably that you should not dig here.
Deep Geologic Repositories (DGR’s)
The most popular of all solutions for long term disposal of radioactive was these day has to be deep geologic repositories. The idea behind this solution is to bury the waste hundreds of metres underground in an engineered space in a geologically impermeable and stable location. Almost every nation around the world with a nuclear program is investigating this means of disposal. This solution, while certainly expensive, meets the above criteria on all counts. The waste will be isolated for geological time scales. The site evaluation will use a wide variety of methods to confirm this. The waste will be recoverable as it is not that deeply buried and is still on land. In fact, some proposals I have seen for these repositories include an underground lab as well so people will actually be working at the repository site. Finally, the hundreds of metres of solid rock and engineered barriers will withstand attempts to access the waste. Most of these site investigations are looking at igneous rock, however, there is a strong case to be made that sedimentary sites may even be better. The current Canadian site under evaluation for low and intermediate level waste is in sedimentary rock and shows excellent potential as an environment that has been isolated for the last 400 million years.
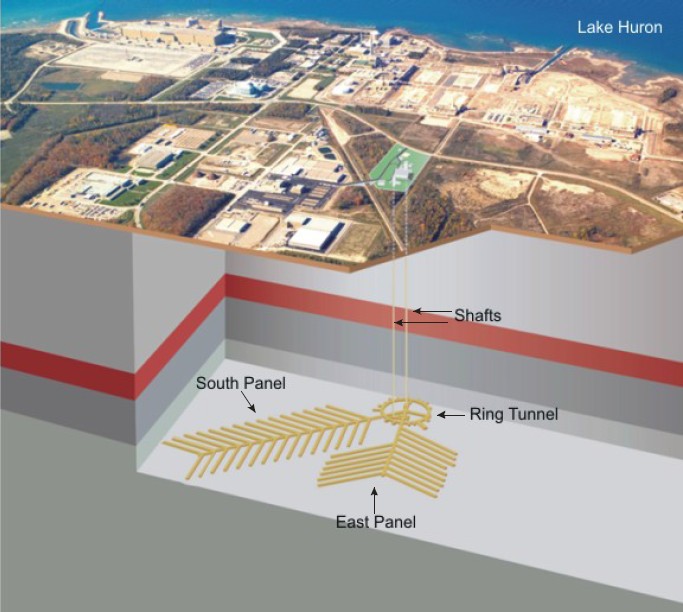
A conceptual diagram for the proposed DGR in southern Ontario – Source: NWMO (2011), Descriptive Geosphere Site Model
Dilution in the ocean/Sub seafloor burial
This option is likely not going to be the most popular in my poll below, but in order to give equal treatment to all of the proposals I feel that it should be mentioned. The basics behind this idea are best described by the old and oft-repeated quote “dilution is the solution to pollution”. In essence, the idea is simple: drop the waste into the deep ocean where it can slowly disperse, decay and dilute. Obviously for high level waste this is a bad idea and is certainly not popular among the international community. However, while it may seem that this idea is ludicrous there are numerous operating nuclear facilities that currently release radioactive isotopes into the ocean. In fact, the nuclear fuel reprocessing facilities of Sellafield and La Hague are allowed to release a certain amount every year. These radioisotopes are then dispersed in the ocean currents and diluted. They are released at extremely low levels to begin with. However, their presence has afforded oceanographers the opportunity to use these isotopes as tracers of ocean currents.
Burial of the waste beneath the ocean floor has also been proposed as an option. It bears a similarity to deep geologic disposal, however, in a more remote and isolated environment. This slant does have some merit as long as the waste could still be recovered, however, the cost would be extremely high build the repository and recover the waste. Furthermore, this solution still has the problem of potentially releasing radionuclides into the ocean only with the added difficulty that fixing a leak would involve working hundreds of metres underwater. That said a leak of a small amount of radionuclides would be diluted within the ocean and would be unlikely to result in significant contamination unless it was very large.
Deep sea trenches/Subduction zones
This option might seem kind of similar to the sub seafloor burial option since trenches and subduction zones are part of the seafloor. However, the idea with this option is that the waste would eventually get subducted into the mantle and return to whence it came. Basically this idea is kind of like composting the waste, which admittedly seems appealing. However, this idea has all of the drawbacks of sub seafloor disposal and would certainly make the waste unrecoverable…at least once it had started its journey through the crust.
Blast it into space
This solution certainly meets the permanently isolate criteria as well as withstanding accidental incursion. 2 out of 3 ain’t bad, right? However, it does fall down when it comes to waste recovery for obvious reasons. This is a solution, that while appealing on the surface never really gained significant traction. One of the biggest reasons for this is the extremely costly nature of shuttle launches. The other major issue is where do we aim the rocket? Towards another planet? Into the vastness of space? Into the sun? All of these options are fraught with difficulties. Finally, if the launch should fail there is a possibility that the waste could be released into the local environment as well.
Disposal in ice sheets
Disposal of radioactive waste in ice sheets has been considered as well, although never very seriously. Continental ice sheets, such as the Antarctic and Greenland ice sheets, have been stable for many thousands of years. Additionally, as high level wastes are heat generating storage in glaciers would provide in situ cooling. Win-Win right? At least that was the early logic on the proposal when it came out the 70’s. In practice, this is clearly a pretty bad idea, especially since as the climate warms the long term stability of continental ice sheets is not as certain as it was when this idea was proposed. Furthermore, the heat generating capability of high level waste does not go away quickly, as the figure above shows, meaning that the waste could potentially melt its way out of the ice sheet or mix with the meltwater that was produced spreading radioisotopes. Thus, ensuring the waste’s stability for millions of years into the future is uncertain at best with this storage solution.

A composite satellite image of Antarctica. Source: Wikimedia Commons
Long term above ground storage
The final choice is store the waste in an engineered facility on the surface of the Earth. Generally, this disposal option is considered temporary or only for low-level radioactive waste. However, it has been investigated as a long term solution as well. The idea is the the waste is placed together in a constructed facility that has been engineered to isolate it from the environment and withstand any attempts at incursion. At the same time the facility would still be easy to access and make waste recovery very simple.One issue with this option is that since the waste is on the Earth surface it is impossible to truly seal it. Thus, future generations would also have to be responsible for maintaining the facility and ensuring the waste remained isolated.

An example of a long term above ground storage facility and its engineered barriers. This facility is planned for low-level waste in Port Hope, Ontario. Source
Ultimately, the legacy of nuclear power generation has to be dealt with in a safe and responsible manner for thousands to millions of years into the future. As a society we recognize this and the ideas outlined above range from the practical to the fantastic. I would like your opinion this question. Which of these solutions do you prefer? If the answer is none of above please comment below and encourage discussion on this topic!
[polldaddy poll=”8130488″]