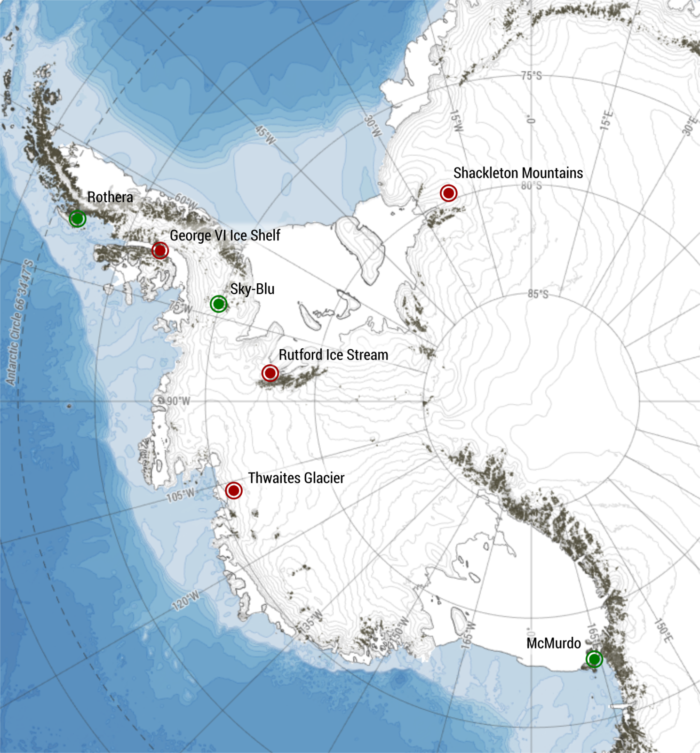
At the beginning of March, just over a month ago, sea ice in the Arctic reached its annual maximum extent. As currently all media attention is focused on other news, you might have missed that, once again, this maximum fell below the 1981 to 2010 average maximum extent. When reading headlines about such sea-ice facts, you may have been confused by the seemingly interchangeable use of “sea-ice exte ...[Read More]
Did you know… the difference between sea-ice area and sea-ice extent?